Concept
Version 9
Created by Boundless
Experimental Determination of Reaction Rates
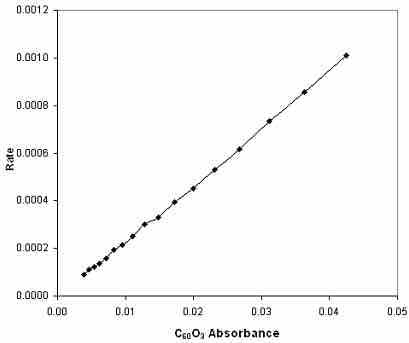
Reaction rate vs. absorbance
The absorbance is directly proportional to the concentration, so this is simply a plot of the rate law, rate = k[C60O3], and the slope of the line is the rate constant, k.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"John S. Hutchinson, Reaction Rates. November 11, 2014."
http://cnx.org/contents/74008f7d-eef5-4708-89fd-0c3fc323acc8@2/Reaction_Rates
OpenStax CNX
CC BY-SA 3.0.