Ionic vs Covalent Bonding
Chemical compounds are frequently classified by the bonds between constituent atoms. There are multiple kinds of attractive forces, including covalent, ionic, and metallic bonds. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are held together by attractive electrostatic forces.
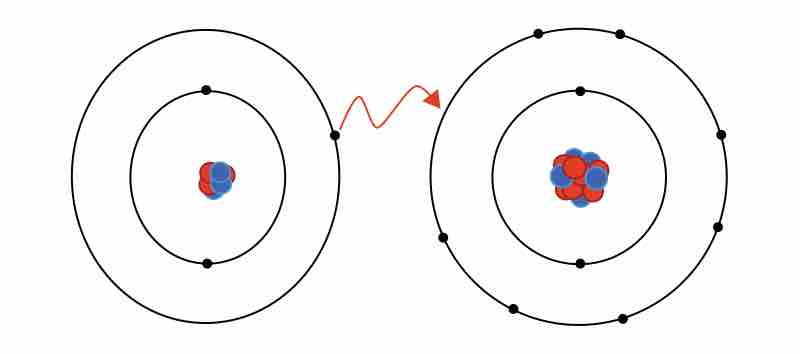
Ionic bonding
The formation of an ionic bond between lithium and fluorine to form LiF.
In reality, the bond between these atoms is more complex than this model illustrates. The bond formed between any two atoms is not a purely ionic bond. All bonding interactions have some covalent character because the electron density remains shared between the atoms. The degree of ionic versus covalent character of a bond is determined by the difference in electronegativity between the constituent atoms. The larger the difference, the more ionic the nature of the bond. In the conventional presentation, bonds are designated as ionic when the ionic aspect is greater than the covalent aspect of the bond. Bonds that fall in between the two extremes, having both ionic and covalent character, are classified as polar covalent bonds. Such bonds are thought of as consisting of partially charged positive and negative poles.
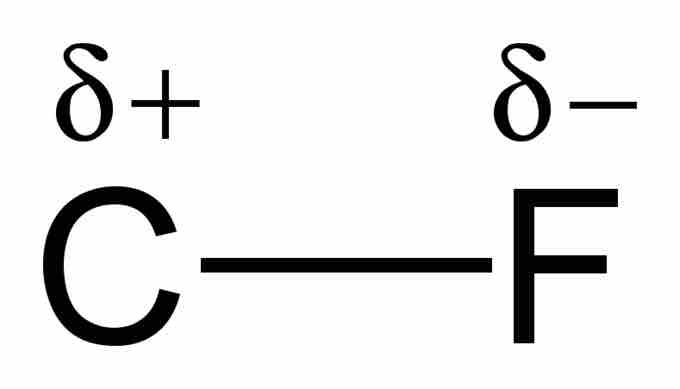
Example of a polar covalent bond
When a carbon atom forms a bond with fluorine, they share a pair of electrons. However, because fluorine is more highly electronegative than carbon, it attracts that shared electron pair closer to itself and thus creates an electric dipole. The lowercase greek delta written above the atoms is used to indicate the presence of partial charges. This bond is considered to have characteristics of both covalent and ionic bonds.
Though ionic and covalent character represent points along a continuum, these designations are frequently useful in understanding and comparing the macroscopic properties of ionic and covalent compounds. For example, ionic compounds typically have higher boiling and melting points, and they are also usually more soluble in water than covalent compounds.