Concept
Version 8
Created by Boundless
Ionic vs Covalent Bond Character
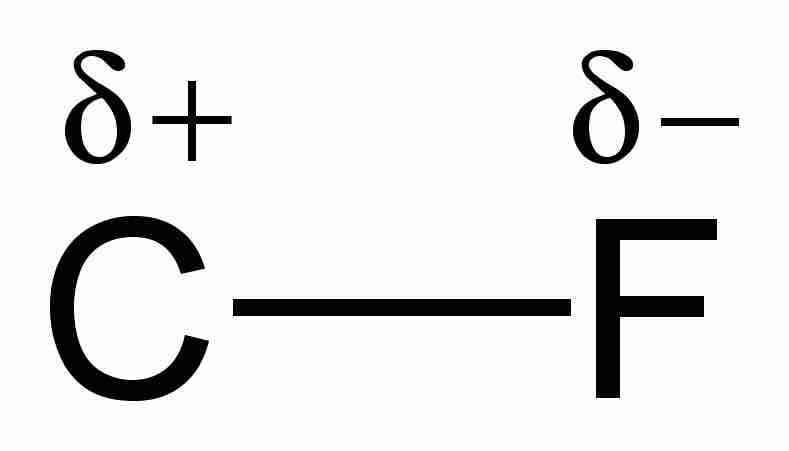
Example of a polar covalent bond
When a carbon atom forms a bond with fluorine, they share a pair of electrons. However, because fluorine is more highly electronegative than carbon, it attracts that shared electron pair closer to itself and thus creates an electric dipole. The lowercase greek delta written above the atoms is used to indicate the presence of partial charges. This bond is considered to have characteristics of both covalent and ionic bonds.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Carbon-fluorine-bond-polarity-2D-black."
https://commons.wikimedia.org/wiki/File:Carbon-fluorine-bond-polarity-2D-black.png
Wikimedia Commons
Public domain.