Determining the Number of Valence Electrons
In order to write the Lewis symbol for an atom, you must first determine the number of valence electrons for that element. The arrangement of the periodic table can help you figure out this information. Since we have established that the number of valence electrons determines the chemical reactivity of an element, the table orders the elements by number of valence electrons.
Each column (or group) of the periodic table contains elements that have the same number of valence electrons. Furthermore, the number of columns (or groups) from the left edge of the table tells us the exact number of valence electrons for that element. Recall that any valence level can have up to eight electrons, except for the first principal energy level, which can only have two.
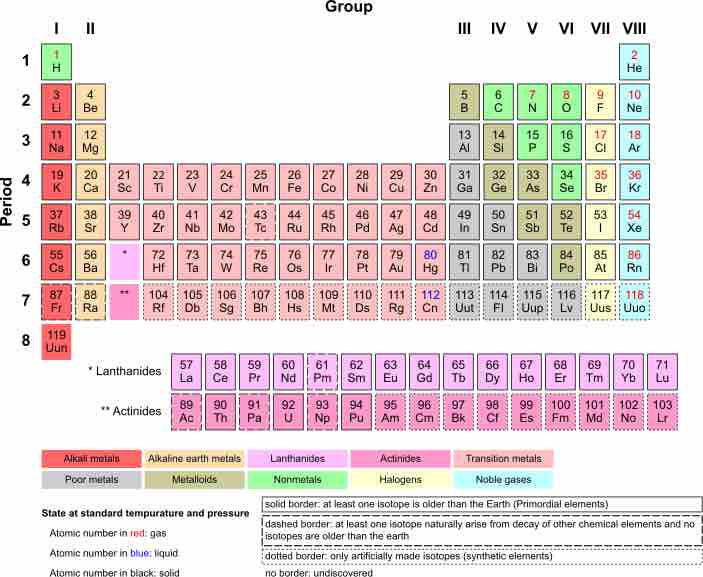
Periodic table of the elements
Group numbers shown by Roman numerals (above the table) tell us how many valence electrons there are for each element.
Some periodic tables list the group numbers in Arabic numbers instead of Roman numerals. In that case, the transition metal groups are included in the counting and the groups indicated at the top of the periodic table have numbers 1, 2, 13, 14, 15, 16, 17, 18. The corresponding roman numerals used are I, II, III, IV, V, VI, VII, VIII.
Survey of the Groups in the Periodic Table
Take the first column or group of the periodic table (labeled 'I'): hydrogen (H), lithium (Li), sodium (Na), potassium (K), etc. Each of these elements has one valence electron. The second column or group (labeled 'II') means that beryllium (Be), magnesium (Mg), calcium (Ca), etc., all have two valence electrons.
The middle part of the periodic table that contains the transition metals is skipped in this process for reasons having to do with the electronic configuration of these elements.
Proceeding to the column labeled 'III', we find that those elements (B, Al, Ga, In, ...) have three valence electrons in their outermost or valence level.
We can continue this inspection of the groups until we reach the eighth and final column, in which the most stable elements are listed. These are all gaseous under normal conditions of temperature and pressure, and are called 'noble gases.' Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their valence level. Therefore, these elements have a full valence level that has the maximum number of electrons possible. Helium (He), at the very top of this column is an exception because it has two valence electrons; its valence level is the first principal energy level which can only have two electrons, so it has the maximum number of electrons in its valence level as well.
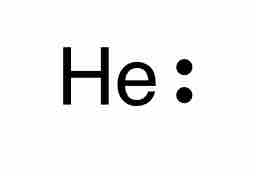
The Lewis symbol for helium
Helium is one of the noble gases and contains a full valence shell. Unlike the other noble gases in Group 8, Helium only contains two valence electrons. In the Lewis symbol, the electrons are depicted as two lone pair dots.
The noble gases represent elements of such stability that they are not chemically reactive, so they can be called inert. In other words, they don't need to bond with any other elements in order to attain a lower energy configuration. We explain this phenomenon by attributing their stability to having a 'full' valence level.
The significance in understanding the nature of the stability of noble gases is that it guides us in predicting how other elements will react in order to achieve the same electronic configuration as the noble gases by having a full valence level.
Writing Lewis Symbols for Atoms
Lewis symbols for the elements depict the number of valence electrons as dots. In accordance with what we discussed above, here are the Lewis symbols for the first twenty elements in the periodic table. The heavier elements will follow the same trends depending on their group.
Once you can draw a Lewis symbol for an atom, you can use the knowledge of Lewis symbols to create Lewis structures for molecules.