In chemistry, valence bond (VB) theory is one of two basic theories—along with molecular orbital (MO) theory—that use quantum mechanics to explain chemical bonding. According to VB theory, a covalent bond forms from the physical overlap of half-filled valence orbitals in two atoms.
Mechanism of Bonding in VB Theory
The VB theory describes the formation of covalent bonds from the overlap of atomic orbitals on two different atoms. Because of the overlap, it is highly probable that a pair of electrons are found in the physical region or space where the orbitals overlap.
Sigma ($\sigma$ ) and Pi ($\pi$ ) Bonds
There are two types of overlapping orbitals: sigma (
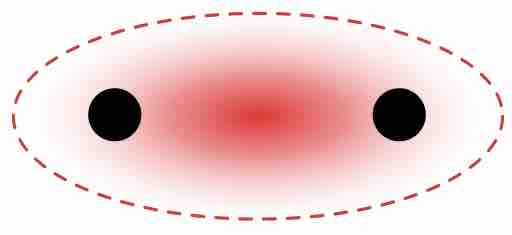
$\sigma$ Bond Formation
Atomic orbitals from two atoms overlap in the region between the nuclei (internuclear axis). Therefore, the resulting electron density of the shared electrons lies in the red region shown in the image.

$\pi$ Bond Formation
Two unhybridized p-orbitals can overlap so that the electron density of the shared electron pair is described by the
Both types of overlapping orbitals can be related to bond order. Single bonds have one sigma bond. Double bonds consist of one
Comparing VB and MO
VB theory complements molecular orbital (MO) theory, which does not adhere to the VB concept that electron pairs are localized between two specific atoms in a molecule. MO theory states that electrons are distributed in sets of molecular orbitals that can extend over the entire molecule. MO theory can predict magnetic and ionization properties in a straightforward manner. VB theory produces similar results, but is more complicated.
Bond Character
An important aspect of the VB theory is the condition of maximum overlap which leads to the formation of the strongest possible bonds. This theory is used to explain the covalent bond formation in many molecules. In the F2 molecule, the F–F
In an HF molecule, the covalent
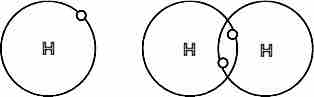
Covalent bond between hydrogen atoms
Each hydrogen atom has one electron. To complete their valence shells, they bond and share one electron with each other. This allows electrons to move about both atoms and gives both atoms access to two electrons; they become a stable H2 molecule joined by a single covalent bond.
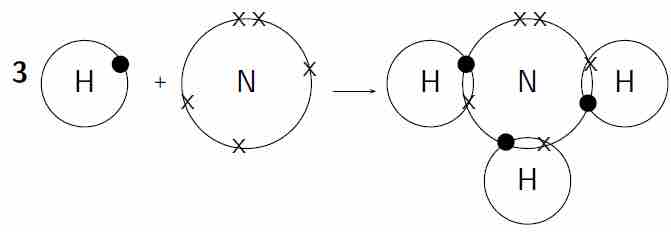
Covalent bonding in a molecule of ammonia
Each hydrogen atom needs one more electron to complete its valence energy shell. The nitrogen atom needs three more electrons to complete its valence energy shell. Therefore, three pairs of electrons must be shared between the four atoms involved. The nitrogen atom will share three of its electrons so that each of the hydrogen atoms now has a complete valence shell. Each of the hydrogen atoms will share its electron with the nitrogen atom to complete its valence shell.