Solubility is Affected by pH
The pH of an aqueous solution can affect the solubility of the solute. By changing the pH of the solution, you can change the charge state of the solute. If the pH of the solution is such that a particular molecule carries no net electric charge, the solute often has minimal solubility and precipitates out of the solution. The pH at which the net charge is neutral is called the isoelectric point, or pI (sometimes abbreviated to IEP).
Using pI to Separate Compounds
As an example, proteins are composed of linked compounds called amino acids. Amino acids all contain the same backbone, which has both an acidic and a basic group. Each amino acid also has a functional group attached to the backbone. These functional groups can be positive, negative, neutral, or polar in nature. The backbone and functional groups give a protein its overall charge. At a pH below the protein's pI, a protein will carry a net positive charge; above its pI, it will carry a net negative charge. Proteins can therefore be separated according to their isoelectric point.
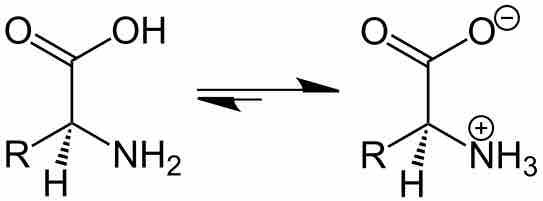
Amino acid backbone as a zwitterion
The backbone of all amino acids contains both acidic (carboxylic acid fragment) and basic (amine fragment) centers. The isomer on the right is a zwitterion. The R indicates where the amino acid specific group is attached.
In a method called isoelectric focusing, proteins are run through a gel that has a pH gradient. The gels are set in a buffer in a container with a negatively charged electrode (cathode) on one end and a positively charged electrode (anode) on the other. When the proteins are added to the solution and current is applied, they migrate toward the electrode with the opposite charge (remember that opposites attract).
For example, a protein that is in a pH region below its isoelectric point will be positively charged and so will migrate towards the cathode (negative charge). As it migrates through a gradient of increasing pH, however, the protein's overall charge will decrease until the protein reaches the pH region that corresponds to its pI. At this point, it has no net charge, and so it stops moving in the gel. The proteins become focused into sharp stationary bands with each protein positioned at a point in the pH gradient corresponding to its pI. This technique is capable of extremely high resolution and can separate proteins that differ by as little as a single charge.
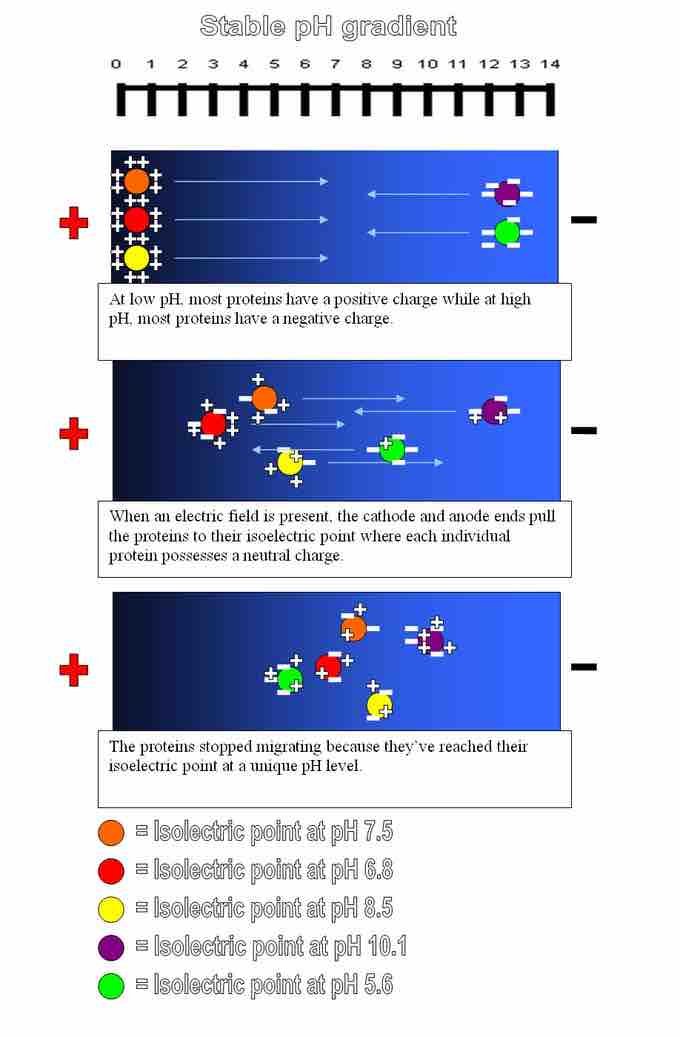
Isoelectric focusing
Overview of an isoelectric focusing experiment to separate proteins based on the differences in their solubilities at different pH values.