Monoprotic Acids
Monoprotic acids are acids able to donate one proton per molecule during the process of dissociation (sometimes called ionization) as shown below (symbolized by HA):
Common examples of monoprotic acids in mineral acids include hydrochloric acid (HCl) and nitric acid (HNO3). On the other hand, for organic acids the term mainly indicates the presence of one carboxylic acid group, and sometimes these acids are known as monocarboxylic acid.
Polyprotic Acids
Polyprotic acid are able to donate more than one proton per acid molecule, in contrast to monoprotic acids that only donate one proton per molecule. Certain types of polyprotic acids have more specific names, such as diprotic acid (two potential protons to donate) and triprotic acid (three potential protons to donate).
For example, oxalic acid, also called ethanedioic acid, is diprotic, having two protons to donate.
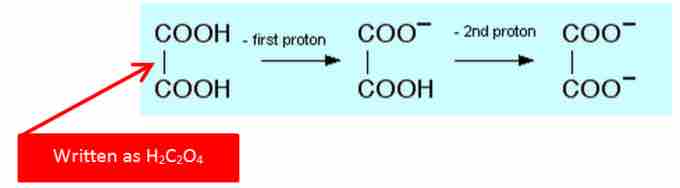
Oxaliic Acid Showing consecutive losses of H+
This image shows how Oxalic Acid will lose two protons in successive dissociations.
If a dilute solution of oxalic acid were titrated with a sodium hydroxide solution, the protons would react in a stepwise neutralization reaction.
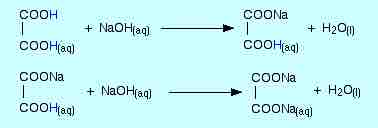
Neutralization of a diprotic acid
Oxalic acid undergoes stepwise neutralization by sodium hydroxide solution.
If the pH of this titration were recorded and plotted against the volume of NaOH added, a very clear picture of the stepwise neutralization emerges, with very distinct equivalence points on the titration curves.
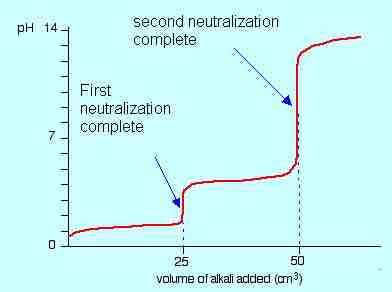
Titration curve for diprotic acid
The titration of dilute oxalic acid with sodium hydroxide (NaOH) shows two distinct neutralization points due to the two protons.
Oxalic acid is an example of an acid able to enter into a reaction with two available protons, having different Ka values for the dissociation (ionization) of each proton.
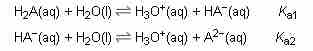
A diprotic acid dissociation
The diprotic acid has two associated values of Ka, one for each proton.
Likewise, a triprotic system can be envisioned. Each reaction proceeds with its unique value of Ka.
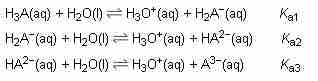
Triprotic acid dissociation
Triprotic acids can make three distinct proton donations, each with a unique Ka.
An example of a triprotic acid is orthophosphoric acid (H3PO4), usually just called phosphoric acid. All three protons can be successively lost to yield H2PO4−, then HPO42-, and finally PO43- the phosphate ion. Another example of a triprotic acid is citric acid, which can successively lose three protons to finally form the citrate ion.