Section 4
Acid-Base Titrations
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
Strong Acid-Strong Base Titrations
A strong acid will react with a strong base to form a neutral (pH = 7) solution.
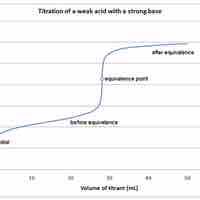
Weak Acid-Strong Base Titrations
A weak acid will react with a strong base to form a basic (pH > 7) solution.
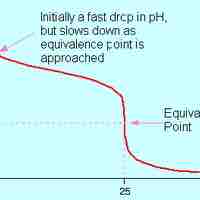
Strong Acid-Weak Base Titrations
A strong acid will react with a weak base to form an acidic (pH < 7) solution.
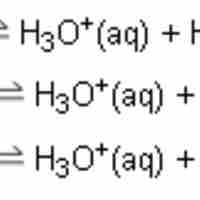
Polyprotic Acid Titrations
Polyprotic acids, also known as polybasic acids, are able to donate more than one proton per acid molecule.
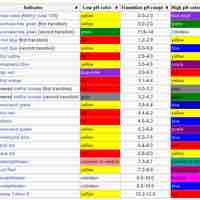
Acid-Base Indicators
An indicator is a weak acid (or a weak base) that has different colors in its dissociated and undissociated states.