Concept
Version 9
Created by Boundless
Acid-Base Indicators
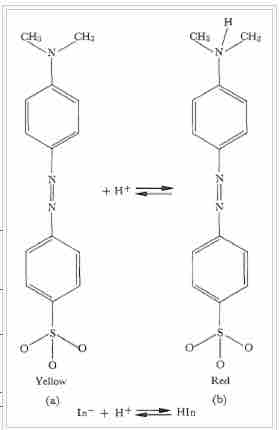
Methyl orange
The molecule methyl orange is commonly used as an indicator in acid-base equilibrium reactions. In base form, on the left in the figure, the color is yellow. Adding a proton yields the structure on the right, colored red. Note that this color change occurs over the pH range from approximately 3-4.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Chemical Principles/Solution Equilibria: Acids and Bases."
http://en.wikibooks.org/wiki/Chemical_Principles/Solution_Equilibria:_Acids_and_Bases%23Indicators
Wikibooks
CC BY-SA 3.0.