X
This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
This article has been viewed 30,905 times.
Many people do not realize that everyday items can be used to do science experiments. Though there are several experiments that you can do from home, one very simple one to start with is the exploding bag. Conduct the experiment using vinegar and baking soda, then change things up a little bit for different results. This will help you understand the science behind why the bag explodes.
Steps
Part 1
Part 1 of 3:
Conducting the Experiment
-
1Select your bag. You can use any plastic bag for this experiment. The preferred method is to use a resealable bag so that you don’t have any air leaks. This will effectively build the pressure inside the bag, eventually causing it to explode.[1]
- You can use other bags and tie them shut, but this can lead to air leaks that bleed off pressure and prevent your bag from exploding.
-
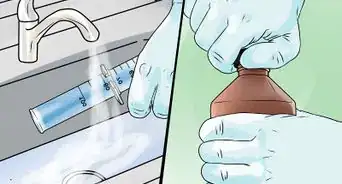
2Pour in vinegar. Add 1 cup (240 ml) of white vinegar to the bag. Vinegar is diluted acetic acid, which is a key ingredient in our explosion. Even though it’s a weak acid, you should still avoid getting it in your eyes or on your skin.[2]
- It is always best practice to wear gloves and goggles when working with any chemicals.
Advertisement -
3Make a baking soda capsule. The moment that baking soda comes in contact with vinegar, a reaction will start. By packing 2 tablespoons (30 ml) of baking soda tightly into a piece of tissue paper and then rolling it up, you create an obstacle between the baking soda and vinegar. This gives you enough time seal the bag and back up a bit.[3]
-
4Add the capsule to the bag. Drop the capsule in the bag, seal the bag, and step away. The moment the capsule goes in, vinegar starts to saturate the tissue paper and contact baking soda. As this happens the paper will degrade and unravel until more and more baking soda reacts. This reaction produces carbon dioxide, which builds up pressure in the bag until the bag explodes.[4]
- You can use these same chemicals to make a cool volcano.
Advertisement
Part 2
Part 2 of 3:
Varying the Parameters
-
1Try a new bag. If you change any aspect of the bag, you change the experiment altogether. See what happens if you use a thicker bag, or a bigger bag. Try using a bag without a seal. Record the differences in each variation in a lab notebook that you can review later.[5]
-
2Dilute the vinegar. Another parameter you can change is the strength of the acid. Try adding half a cup of vinegar and half a cup of water. Notice how it affects the speed of the reaction. You can reduce the strength of the vinegar even more by adding more water. Write each variation down in your notebook for later comparison.[6]
-
3Change the capsule. The reaction cannot start until the vinegar gets to the baking soda. You can change how quickly this happens by changing your capsule. Try a capsule made out of a paper bag, or notebook paper. Write down any ways that the experiment changes as a result.[7]
- Try wrapping the baking soda in plastic wrap and sealing it well. How does this affect the experiment?
-
4Add some color. An exploding bag is always more fun if it slings color into the air. If you want to make your bag pop with different colors, add a few drops of food coloring to the vinegar. This will change the color of the foam that oozes out of the bag after it explodes.[8]
- It's best for cleanup purposes to do this experiment outside, especially if you're adding food coloring.
Advertisement
Part 3
Part 3 of 3:
Understanding the Explosion
-
1Learn about acid-base reactions. When acids and bases come in contact with each other, they undergo what is called a neutralization reaction. Since acids have plenty of extra protons, and bases readily accept protons, they balance each other out chemically. This is precisely what happens when vinegar and baking soda interact. The result is salty water and a release of carbon dioxide.[9]
-
2Study how gases act under pressure. When more gas is created in a closed system, the pressure increases. What this means is that the gases push harder and harder on the outside walls of the container. Higher temperatures will also increase the pressure of the gas by exciting the gas molecules and causing them to bounce more rapidly against the container.
-
3Consider the elastic limits of plastic bags. With more and more gas being created by the vinegar and baking soda reaction, the pressure inside the bag keeps building. Eventually, the force that the gas exerts on the bag will reach what is known as the elastic limit. This is the maximum extent to which the bag may be stretched without permanently altering the size or shape.
- When the bag reaches the elastic limit, the weakest part of the bag will pop or tear open to allow the gas to escape and relieve the pressure.
Advertisement
Warnings
- Don't hold the bag in your hand after adding the baking soda!⧼thumbs_response⧽
- Never throw the bag at cars or property as it can be considered vandalism.⧼thumbs_response⧽
Advertisement
Things You'll Need
- Plastic sandwich bag
- Baking soda
- Vinegar
References
- ↑ https://sciencebob.com/the-exploding-lunch-bag/
- ↑ http://www.kidspot.com.au/things-to-do/activities/exploding-bag-experiment
- ↑ https://sciencebob.com/the-exploding-lunch-bag/
- ↑ http://www.kidspot.com.au/things-to-do/activities/exploding-bag-experiment
- ↑ https://www.exploratorium.edu/science_explorer/bubblebomb.html
- ↑ https://www.exploratorium.edu/science_explorer/bubblebomb.html
- ↑ https://www.exploratorium.edu/science_explorer/bubblebomb.html
- ↑ http://handsonaswegrow.com/kids-experiment-vinegar-baking-soda/
- ↑ http://www.chem4kids.com/files/react_acidbase2.html
About This Article
Advertisement