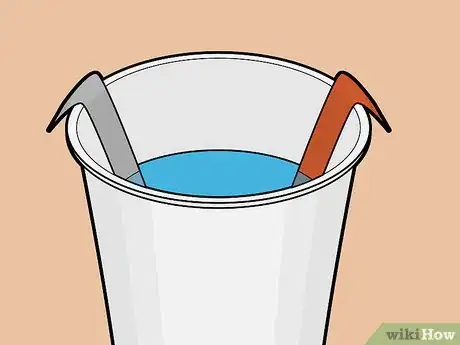wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 41 people, some anonymous, worked to edit and improve it over time.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 23 testimonials and 82% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 989,830 times.
Learn more...
To make your own battery at home, all you need is two different types of metal, some copper wires, and a conductive material. Many household items can be used as the conductive material into which you place your metals — for example, saltwater, a lemon, or even dirt.
This battery creates electricity because the soda acts as an electrolyte for the copper strip and the aluminum strip.
Steps
Making a Soda-powered Battery
-
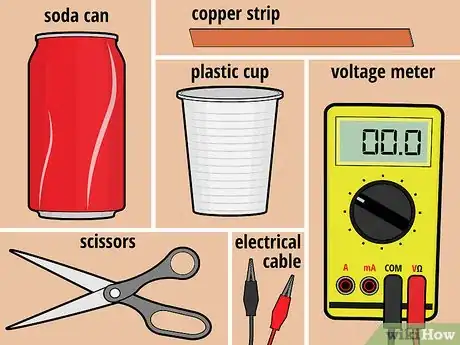
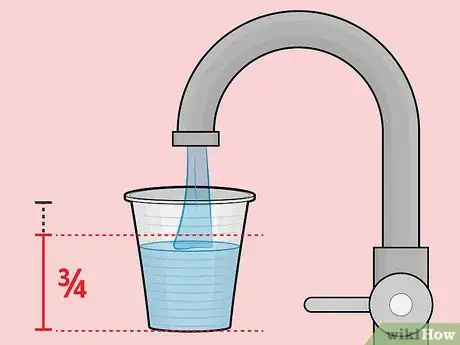
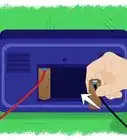
1Gather your materials. For this battery, you will need one unopened can of soda (any type will do), one plastic cup (6 to 8 ounces), and one 3/4-inch-wide strip of copper that's slightly longer than the height of the cup. In addition, you'll need a pair of scissors, a voltage meter, and two electrical lead wires with alligator clips at both ends.[1]
- If you don't already have these materials around your house, you can purchase them at a hardware store nearby.
- You can substitute the copper strip with several pieces of copper wire stuck together or bent in a zig-zag fashion to reach the desired width.
-
2Fill the plastic cup roughly 3/4 full with soda. Note that the cup doesn't absolutely have to be plastic.[2] It just has to be non-metallic. Styrofoam and paper cups will also work if you don't have that.Advertisement
-
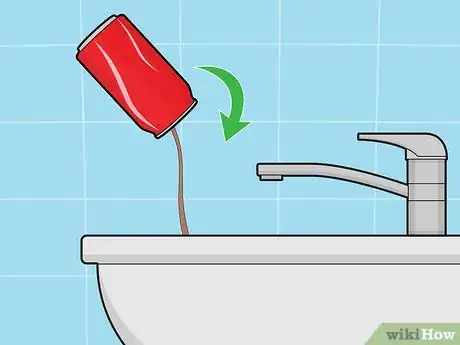
3Make sure the soda can is completely empty. Discard (or drink) any soda that remains in the can. Turn it upside down in the sink and give it a few extra shakes to make sure that all the soda is out.
-
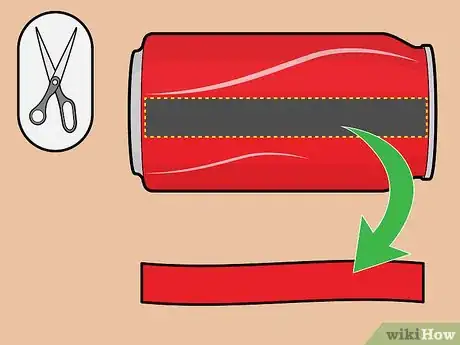
4Cut a strip of aluminum from the soda can. Cut a 3/4-inch-wide strip from the side of the soda can. Ensure that's it's slightly longer than the plastic cup's height; if this isn't possible, don't worry — you can just bend the top of the strip and let it hang over the edge of the cup and into the fluid.
- Instead of cutting the can, you may purchase aluminum strips from a hardware store.
- Aluminum foil is not an effective replacement for an aluminum strip; don't use it!
-
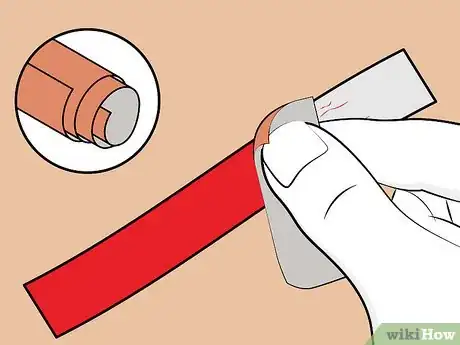
5Sand the aluminum strip (optional). You can skip this step if you bought the aluminum from a hardware store. If you cut the strip from a soda can, you'll need to sand off the coatings (i.e. paint, plastic) on both sides of the strip.
-
6Place the strips into the solution. Ensure that the strips do not touch each other. Place them across from each other — not side by side or overlapping — in the cup.
- Ideally you've cut the strips long enough that their tops sit above the soda, extending slightly past the rim of the cup.
- If the strips do not extend past the cup's rim, you can bend each strip lightly so that it hangs off the edge of the cup.
-
7Attach lead wires to metal strips. Attach one lead wire to one metal strip by opening the alligator clip and closing it on the strip. Then, attach a different lead wire to the other metal strip, again using the alligator clip.
- Be careful not to let the alligator clips touch the soda.
- It doesn't matter which color of wire attaches to which strip.
-
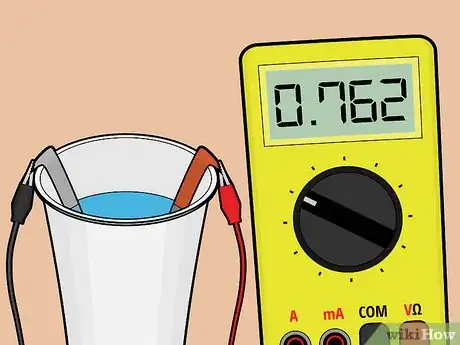
8Test the battery. Following the instructions that come with your voltage meter, connect a lead wire from each metal strip to the voltage meter. The meter should read your battery's voltage at roughly 3/4 of a volt.
Making a Saltwater-powered Battery
-
1Gather your materials. For this battery, you'll need one plastic cup (6 to 8 ounces), two 3/4-inch-wide strips of metal that are taller than the cup, and one tablespoon (14.79 ml) of salt. Each strip must be a different type of metal, but you can choose which type: zinc, aluminum, and copper are popular choices. In addition, you'll need a pair of scissors, a voltage meter, and 2 electrical lead wires with alligator clips at both ends.
- A variation on this recipe is to add one teaspoon (4.93 ml) of salt, one teaspoon (4.93 ml) of vinegar, and a few drops of bleach to the water instead of one tablespoon (14.79 ml) of salt. If you choose this variation, be careful, as bleach is a hazardous chemical.
- Metal strips, electrical lead wires, and voltage meters are available at hardware stores. You should also be able to find lead wires at shops that sell electrical components.
-
2Fill a plastic cup 3/4-full with water. Note that the cup doesn't absolutely have to be plastic. It just has to be non-metallic. Styrofoam and paper cups will also work.
-
3Add 1 tablespoon (14.79 ml) of salt to the water and stir. It's the same process if you decide to follow the salt, vinegar, and bleach variation.
-
4Place the two metal strips into the cup. Ensure that the strips are touching the saltwater and extending past the cup's rim.[3] If the strips are too short, bend them so that they hang off the cup's rim and dip into the solution.
-
5Attach the lead wires to the metal strips. Attach one lead wire to one metal strip using an alligator clip. Then, attach a different lead wire to the other metal strip, again using the alligator clip.
- Be careful not to let the alligator clips touch the water.
- It doesn't matter which color attaches to which strip.
-
6Test the battery. Following the instructions that come with your voltage meter, connect a lead wire from each metal strip to the voltage meter.[4] The meter should read your battery's voltage at roughly 3/4 of a volt.
Making 14-cell Water-powered Battery
-
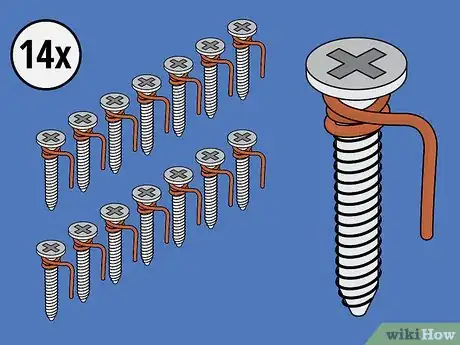
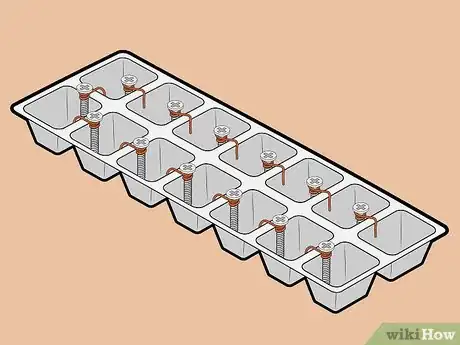
1Gather your materials. For this battery, you'll need some copper wire, 15 sheet metal screws, an ice cube tray, and water.[5] In addition, you'll need a pair of scissors, a voltage meter, and two electrical lead wires with alligator clips at both ends. You'll wrap each screw in copper except for one, which you will use as a negative terminal (to which you will attach one of the lead wires once the battery is complete).
- How many screws you use will depend on how many ice cubes your tray is meant to hold. The tray in this example can hold 14 ice cubes.
- As long as they aren't copper, you can use any type of metal screw. Zinc-coated (galvanized) or aluminum work well.[6] As for size, aim for screws that are around one inch in length.
-
2Wrap copper wire around 14 of the 15 screws. Wrap a piece of copper wire two times around the top of each screw, just beneath its head. After wrapping the wire around a screw, use your finger to bend the wire into a hook. You'll use this hook to clip the screw to the edge of its ice cube tray compartment.
- You can either pre-cut the copper wire into pieces long enough to wrap around each screw (with a bit extra for the hooks), or you can work with it long, cutting it as you finish each screw.
-
3Attach one screw to each ice cube tray compartment. Each ice cube hole will act as a single cell in your battery. Affix one screw to the edge of each cell. Ensure that there is only one screw in each cell.
-
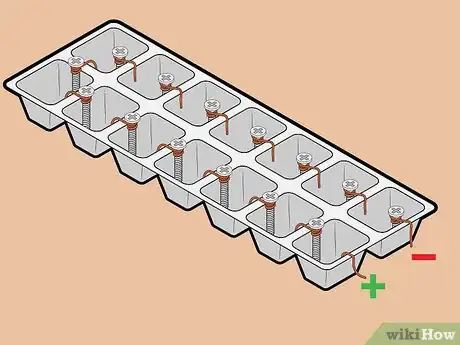
4Attach positive and negative terminals to one end of the tray. On one end of the tray, hook a piece of copper wire to the outer edge of one of the cells. On the same end of the tray, place a screw in the cell next to the one in which you just placed the copper wire. Ensure that the screw sits above the rim of the tray, as you will need to attach a lead wire to it.
-
5Fill each cell with water. Ensure that the cells are full enough that the copper wire hooks and screws are touching the water.
-
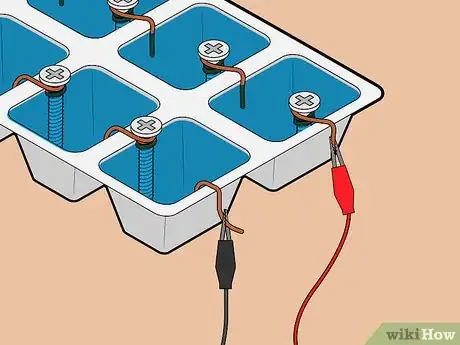
6Attach lead wires to positive and negative terminals. Attach one lead wire to the copper wire terminal using its alligator clip. Then, attach a different lead wire to the screw terminal, again using its alligator clip.
- Be careful not to let the alligator clips touch the water.
- It doesn't matter which color attaches to which terminal.
-
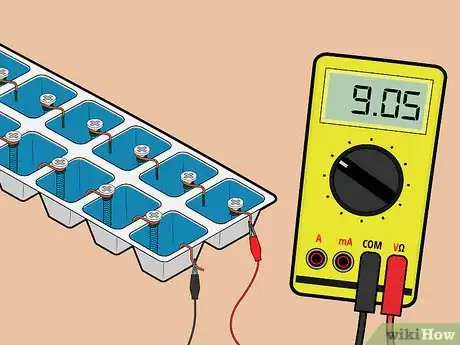
7Test the battery. Attach the other ends of the lead wires to your voltage meter. The 14-cell battery that you've just made should measure roughly 9 volts.
-
8Boost the voltage. You can boost the voltage of your battery by switching your conductive solution to saltwater, vinegar, bleach, lemon juice, or lime juice, or by using more copper.[7]
Making a Hand-powered Battery
-
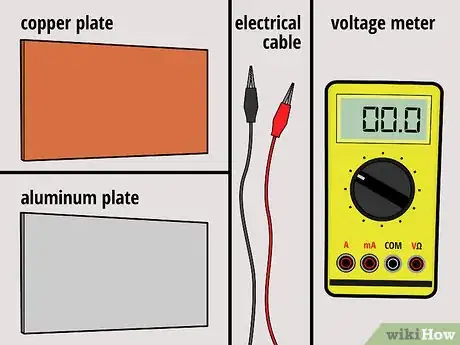
1Gather your materials. For this battery, you'll need one copper plate and one aluminum plate -- both roughly the size of your hands. You'll also need two electrical lead wires with alligator clips at both ends, and you'll need a voltage meter.
- You can purchase the metal plates, wires, and voltage meter at a hardware store.
-
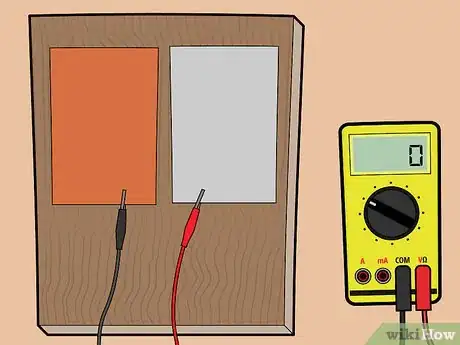
2Place the aluminum and copper plates on a piece of wood. If you don't have a piece of wood, you can also place the plates on another non-metallic surface — for example, plastic.
-
3Connect the plates to a voltage meter. Using the alligator clips, connect the copper sheet to one end of the voltage meter, and the aluminum sheet to the other end of the voltage meter.
- If you're unsure of how to connect items to your specific voltage meter, check its instruction manual.
-
4Place one hand on each plate. When you place your hands on the metal plates, the sweat on your hands should react with the metal plates to produce a reading on the voltage meter.
- If the meter doesn't show a reading, reverse your connections: attach the copper plate to the terminal that the aluminum plate was connected to, and vice versa.
- If you still struggle to get a reading, check the connection and wiring. If everything is working well, it could be that the plates are oxidized. To remove oxidization, clean the plates with a pencil eraser or steel wool.
Community Q&A
-
QuestionWhat in the Coke is attracted to the copper and aluminum?
 Community AnswerThe Coke contains phosphoric acid, which acts as an electrolyte. The electrolyte act as a barrier to the electrons, forcing them to go through one wire, through the device, thorough the other wire and back to the opposite end of the battery, all in an attempt to create a state of equilibrium with the negative and positive charges.
Community AnswerThe Coke contains phosphoric acid, which acts as an electrolyte. The electrolyte act as a barrier to the electrons, forcing them to go through one wire, through the device, thorough the other wire and back to the opposite end of the battery, all in an attempt to create a state of equilibrium with the negative and positive charges. -
QuestionFor the experiments, why use soda, lemon juice, or vinegar?
 Community AnswerThey make the voltage higher. When the liquid's pH is farther away from neutral (7), they make more electricity.
Community AnswerThey make the voltage higher. When the liquid's pH is farther away from neutral (7), they make more electricity. -
QuestionWhat is the science behind a salt water battery?
 Community AnswerOne of the main components of a battery is an electrolyte. Electrolytes can be found in sodium ions, which compose salt.
Community AnswerOne of the main components of a battery is an electrolyte. Electrolytes can be found in sodium ions, which compose salt.
References
- ↑ http://sci-toys.com/scitoys/scitoys/echem/batteries/batteries.html
- ↑ https://www.greenoptimistic.com/coke-can-battery/
- ↑ https://www.scholastic.com/parents/school-success/learning-toolkit-blog/kid-maker-how-to-make-salt-water-battery.html
- ↑ https://www.scholastic.com/parents/school-success/learning-toolkit-blog/kid-maker-how-to-make-salt-water-battery.html
- ↑ https://www.youtube.com/watch?v=aaMWBhRcqew
- ↑ https://www.youtube.com/watch?v=aaMWBhRcqew
- ↑ https://www.youtube.com/watch?v=aaMWBhRcqew
- ↑ http://www.survival-manual.com/homemade-battery.php
About This Article
To make a homemade battery, start by filling a non-metal cup almost all the way with canned soda. Next, cut a 3/4-inch-wide strip of aluminum from the side of the soda can and place it into the soda. Situate a copper strip purchased from a hardware store in the soda on the opposite side of the cup. Then, attach lead wires to the 2 metal strips to test your battery with a voltage meter! To learn how to make a homemade battery with salt water, read on!