
Dipylidium caninum
[Dipylidium caninum]
Causal Agent
Dipylidium caninum (the double-pored dog tapeworm) mainly infects dogs and cats, but is occasionally found in humans.
Life Cycle

Gravid proglottids are passed intact in the feces or emerge from the perianal region of the host  . Subsequently they release typical egg packets
. Subsequently they release typical egg packets  . On rare occasions, proglottids rupture and egg packets are seen in stool samples. Following ingestion of an egg by the intermediate host (larval stages of the dog or cat flea Ctenocephalides spp.), an oncosphere is released into the flea's intestine. The oncosphere penetrates the intestinal wall, invades the insect's hemocoel (body cavity), and develops into a cysticercoid larva
. On rare occasions, proglottids rupture and egg packets are seen in stool samples. Following ingestion of an egg by the intermediate host (larval stages of the dog or cat flea Ctenocephalides spp.), an oncosphere is released into the flea's intestine. The oncosphere penetrates the intestinal wall, invades the insect's hemocoel (body cavity), and develops into a cysticercoid larva  . The larva develops into an adult, and the adult flea harbours the infective cysticercoid
. The larva develops into an adult, and the adult flea harbours the infective cysticercoid  . The vertebrate host becomes infected by ingesting the adult flea containing the cysticercoid
. The vertebrate host becomes infected by ingesting the adult flea containing the cysticercoid  . The dog is the principal definitive host for Dipylidium caninum. Other potential hosts include cats, foxes, and humans (mostly children)
. The dog is the principal definitive host for Dipylidium caninum. Other potential hosts include cats, foxes, and humans (mostly children)  ,
,  . Humans acquire infection by ingesting the cysticercoid contaminated flea. This can be promulgated by close contact between children and their infected pets. In the small intestine of the vertebrate host the cysticercoid develops into the adult tapeworm which reaches maturity about 1 month after infection
. Humans acquire infection by ingesting the cysticercoid contaminated flea. This can be promulgated by close contact between children and their infected pets. In the small intestine of the vertebrate host the cysticercoid develops into the adult tapeworm which reaches maturity about 1 month after infection  . The adult tapeworms (measuring up to 60 cm in length and 3 mm in width) reside in the small intestine of the host, where they each attach by their scolex. They produce proglottids (or segments) which have two genital pores (hence the name "double-pored" tapeworm). The proglottids mature, become gravid, detach from the tapeworm, and migrate to the anus or are passed in the stool
. The adult tapeworms (measuring up to 60 cm in length and 3 mm in width) reside in the small intestine of the host, where they each attach by their scolex. They produce proglottids (or segments) which have two genital pores (hence the name "double-pored" tapeworm). The proglottids mature, become gravid, detach from the tapeworm, and migrate to the anus or are passed in the stool  .
.
Geographic Distribution
Worldwide. Human infections have been reported in Europe, the Philippines, China, Japan, Argentina, and the United States.
Clinical Presentation
Most infections with Dipylidium caninum are asymptomatic. Pets may exhibit behavior to relieve anal pruritis (such as scraping anal region across grass or carpeting). Mild gastrointestinal disturbances may occur. The most striking feature in animals and children consists of the passage of proglottids. These can be found in the perianal region, in the feces, on diapers, and occasionally on floor covering and furniture. The proglottids are motile when freshly passed and may be mistaken for maggots or fly larvae.
Dipylidium caninum egg packets in wet mounts.
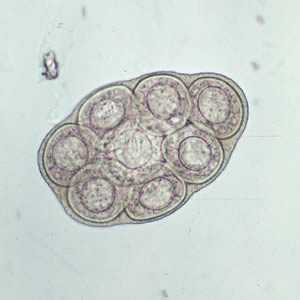
Figure A: D. caninum egg packet, containing 8 visible eggs, in a wet mount.

Figure B: D. caninum egg packet in a wet mount.
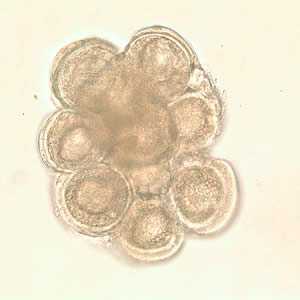
Figure C: D. caninum egg packet in wet mount.

Figure D: D. caninum egg packet in wet mount.
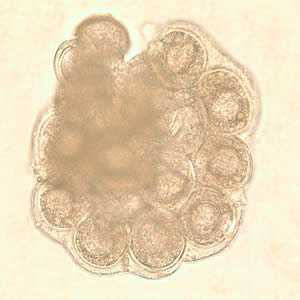
Figure E: D. caninum egg packet in wet mount.
D. caninum eggs in wet mounts under conventional and differential interference contrast microscopy.
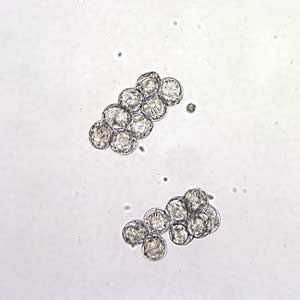
Figure A: D. caninum eggs clumped together in a wet mount. Image taken at 200x magnification.
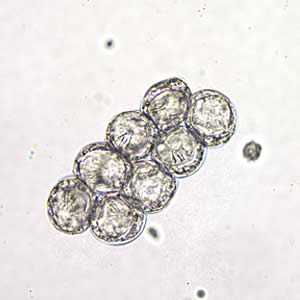
Figure B: D. caninum eggs clumped together in a wet mount. Image taken at 400x magnification, hooklets in the some of the eggs are visible.
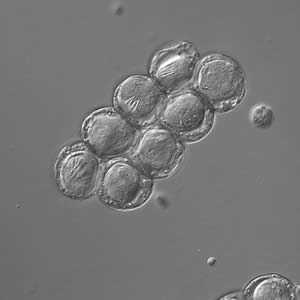
Figure C: D. caninum eggs clumped together under differential interference contrast microscopy (same eggs as in Image B).

Figure D: Close up of Image C. Note the visible hooklets in three of the eggs.
D. caninum proglottids.
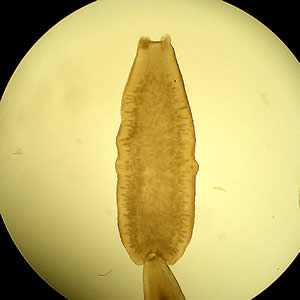
Figure A: D. caninum proglottid under a dissecting microscope cleared with lactophenol.

Figure B: D. caninum proglottid.
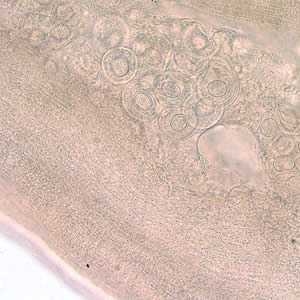
Figure C: D. caninum proglottid partially cleared with lactophenol, showing eggs and egg packets.
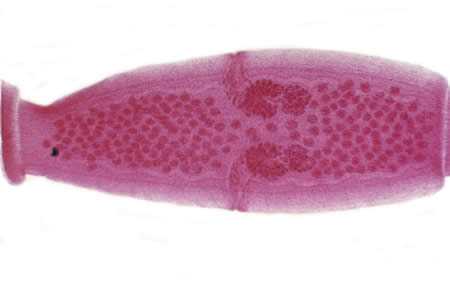
Figure D: D. caninum proglottid. The genital pores are clearly visible in the carmine-stained proglottid.
Cross-section of a D. caninum proglottid stained with hematoxylin and eosin (H&E).
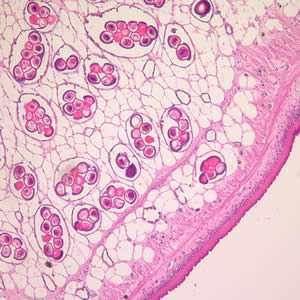
Figure A: Cross-section of a D. caninum proglottid stained with H&E. Image taken at 100x magnification.
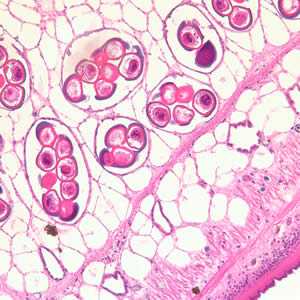
Figure B: Cross-section of a D. caninum proglottid stained with H&E. Image taken at 200x magnfication.
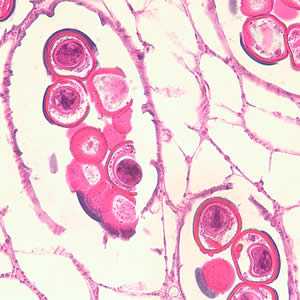
Figure C: Cross-section of a D. caninum proglottid stained with H&E. Image taken at 400x magnification
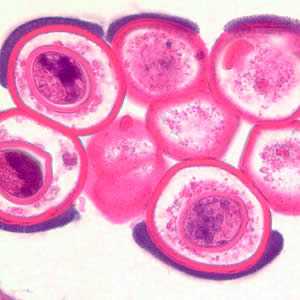
Figure D: Cross-section of a D. caninum proglottid stained with H&E. Image taken at 1000x magnification.
D. caninum scolex.

Figure A: D. caninum scolex.
Adult tapeworm of D. caninum.

Figure A: Adult tapeworm of D. caninum. The scolex of the worm is very narrow and the proglottids, as they mature, get larger.
Diagnostic Findings
The diagnosis is made by demonstrating the typical proglottids or egg packets in the stool or the environment.
Treatment Information
Praziquantel, adults, 5-10 mg/kg orally in a single-dose therapy. Praziquantel is not approved for treatment of children less than 4 years old but this drug has been used successfully to treat cases of D. caninum infection in children as young as 6 months.
Niclosamide is effective but is unvailable in the United States. No purge or follow-up stool examination is indicated, but appearance of proglottids after therapy is indication for retreatment. The infection is self-limiting in the human host and typically spontaneously clears by 6 weeks.
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
Niclosamide
Niclosamide is NOT available for human use in the United States.
Note on Treatment in Pregnancy
References
- Mackoviak PA. A Neonate with worms. Clin Infect Dis 2008;46:1145, 1786-88.
- Samkari A, Kiska DL, Riddell SW et al. Dipylidium caninum mimicking recurrent Enterobius vermicularis (pinworm) infection. Clin Pediatr (Phila) 2008;47:397-9.
- Molina C, Ogburn J, Adegboyega P. Infection by Dipylidium caninum in an infant. Arch Pathol Lab Med 2003;127:e157-9.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir