The effects of changes in volume and pressure on chemical equilibrium can be predicted using Le Chatelier's Principle. Le Chatelier's Principle states that disturbances to a system in equilibrium can be predicted: opposing shifts in the system will occur to restore equilibrium. This principle can be applied to changes in temperature, concentration, volume, and pressure.
One example of the effect of changing volume is shown in . As can be seen, a reduction in volume yields an increase in the pressure of the system, because volume and pressure are inversely related.
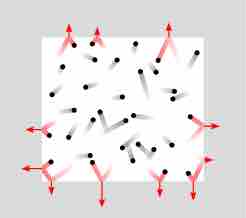
Pressure
A schematic of the pressure due to the collisions of gas particles with the walls of a vessel
Le Chatelier's Principle can be used to predict the response of a reversible chemical reaction to a change in the system. In order to compensate for the increasing pressure and decreasing volume of the system, the new equilibrium state will favor conditions that decrease the pressure in the system. One result that can be predicted using this principle is that reactions that result in an increased number of moles of gas will be pushed in the reverse direction under increased pressure. The same reactions will be pushed in the forward direction -- that is to say, toward product formation -- under conditions of decreased pressure or increased volume. The opposite predictions will hold for reactions that result in a decreased number of moles of gas. If the number of moles of gas produced is equal for the forward and reverse reactions, then a change in pressure or volume will have no effect on the equilibrium state of the reaction.