Anion Regulation
Excretion of ions occurs mainly through the kidneys, with lesser amounts lost in sweat and in feces. In addition, excessive sweating may cause a significant loss, especially of the anion chloride. Severe vomiting or diarrhea will also cause a loss of chloride and bicarbonate ions. Adjustments in respiratory and renal functions allow the body to regulate the levels of these ions in the extracellular fluid (ECF).
Chloride
Chloride is the predominant extracellular anion and is a major contributor to the osmotic pressure gradient between the intracellular fluid (ICF) and extracellular fluid (ECF). Chloride also plays an important role in maintaining proper hydration and functions to balance cations in the ECF, maintaining the electrical neutrality of this fluid. The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions.
Hypochloremia, or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia. In contrast, hyperchloremia, or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt (NaCl) or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis. In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels; therefore, analysis of sweat is often used in the diagnosis of the disease.
Bicarbonate
Bicarbonate is the second most abundant anion in the blood. Its principal function is to maintain your body's acid-base balance by being part of buffer systems. Bicarbonate ions result from a chemical reaction that starts with carbon dioxide (CO2) and water - two molecules that are produced at the end of aerobic metabolism. Only a small amount of CO2 can be dissolved in body fluids; thus, over 90 percent of the CO2 is converted into bicarbonate ions, HCO3–, through the following reactions:
CO2 + H2O ↔ H2CO3 ↔ H2CO3- + H+
The bidirectional arrows indicate that the reactions can go in either direction depending on the concentrations of the reactants and products. Carbon dioxide is produced in large amounts in tissues that have a high metabolic rate and is converted into bicarbonate in the cytoplasm of red blood cells through the action of an enzyme called carbonic anhydrase. Bicarbonate is transported in the blood, and once in the lungs, the reactions reverse direction, and CO2 is regenerated from bicarbonate to be exhaled as metabolic waste .
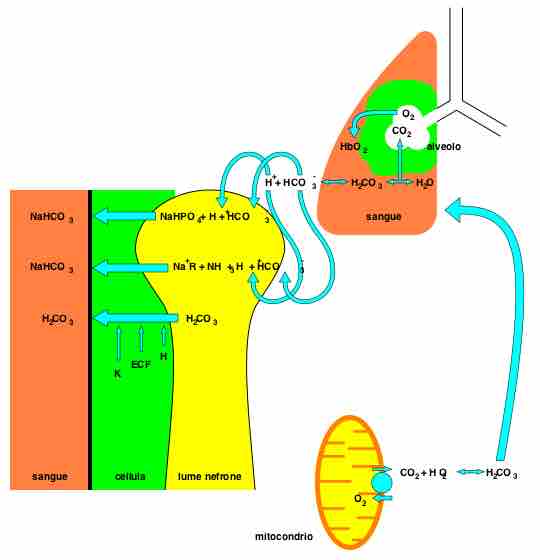
Bicarbonate as a Buffering System
In the lungs, CO2 is produced from bicarbonate and removed as metabolic waste through the reverse reaction of the bicarbonate bidirectional equation.
Phosphate
Phosphate is present in the body in three ionic forms: H2PO4−, HPO42−, and PO43−.
The addition and removal of phosphate from proteins in all cells is a pivotal strategy in the regulation of metabolic processes. Phosphate is useful in animal cells as a buffering agent, and the most common form is HPO2−4. Bone and teeth bind up 85 percent of the body's phosphate as part of calcium-phosphate salts. In addition, phosphate is found in phospholipids, such as those that make up the cell membrane, and in ATP, nucleotides, and buffers.
Hypophosphatemia, or abnormally low phosphate blood levels, occurs with heavy use of antacids, during alcohol withdrawal, and during malnourishment. In the face of phosphate depletion, the kidneys usually conserve phosphate, but during starvation, this conservation is impaired greatly. Hyperphosphatemia, or abnormally increased levels of phosphates in the blood, occurs if there is decreased renal function or in cases of acute lymphocytic leukemia. Additionally, because phosphate is a major constituent of the ICF, any significant destruction of cells can result in dumping of phosphate into the ECF.