Concept
Version 8
Created by Boundless
Humidity, Evaporation, and Boiling
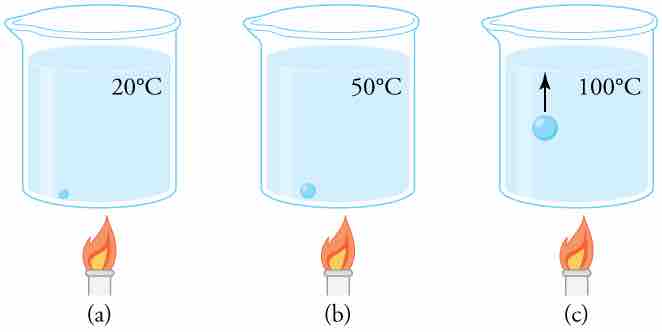
Close-up of the Boiling Process
(a) An air bubble in water starts out saturated with water vapor at 20ºC. (b) As the temperature rises, water vapor enters the bubble because its vapor pressure increases. The bubble expands to keep its pressure at 1.00 atm. (c) At 100ºC, water vapor enters the bubble continuously because water's vapor pressure exceeds its partial pressure in the bubble, which must be less than 1.00 atm. The bubble grows and rises to the surface.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. November 3, 2012."
http://cnx.org/content/m42219/latest/?collection=col11406/1.7
OpenStax CNX
CC BY 3.0.