Diffusion is the movement of particles move from an area of high concentration to an area of low concentration until equilibrium is reached . A distinguishing feature of diffusion is that it results in mixing or mass transport without requiring bulk motion. Thus, diffusion should not be confused with convection or advection, which are other transport mechanisms that use bulk motion to move particles from one place to another.
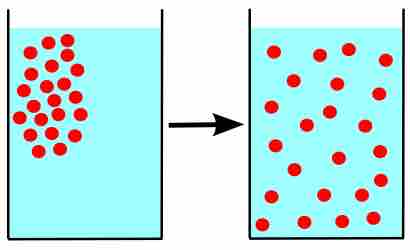
Diffusion
Particles moving from areas of high concentration to areas of low concentration.
Molecular diffusion, often called simply diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. However, diffusion can still occur in the absence of a concentration gradient.
The result of diffusion is a gradual mixing of material. In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.