Beta decay is a type of radioactive decay in which a beta particle (an electron or a positron) is emitted from an atomic nucleus, as shown in . Beta decay is a process that allows the atom to obtain the optimal ratio of protons and neutrons.
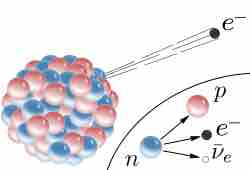
Beta Decay
β decay in an atomic nucleus (the accompanying antineutrino is omitted). The inset shows beta decay of a free neutron
There are two types of beta decay. Beta minus (β) leads to an electron emission (e−); beta plus (β+) leads to a positron emission (e+). In electron emission an electron antineutrino is also emitted, while positron emission is accompanied by an electron neutrino. Beta decay is mediated by the weak force.
Emitted beta particles have a continuous kinetic energy spectrum, ranging from 0 to the maximal available energy (Q), that depends on the parent and daughter nuclear states that participate in the decay. The continuous energy spectra of beta particles occur because Q is shared between a beta particle and a neutrino. A typical Q is around 1 MeV, but it can range from a few keV to a several tens of MeV. Since the rest mass energy of the electron is 511 keV, the most energetic beta particles are ultrarelativistic, with speeds very close to the speed of light.
Since the proton and neutron are part of an atomic nucleus, beta decay processes result in transmutation of one chemical element into another. For example:
137Cs
11Na
Beta decay does not change the number of nucleons, A, in the nucleus; it changes only its charge, Z. Therefore the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay.
A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a few exceptions with half-lives so long that they have not had enough time to decay since the moment of their nucleosynthesis. One example is the odd-proton odd-neutron nuclide 40 K, which undergoes both types of beta decay with a half-life of 1.277 ·109 years.