X-radiation (composed of x-rays) is a form of electromagnetic radiation. X-rays have wavelengths in the range of 0.01 to 10 nanometers, which corresponds to frequencies in the range of 30 petahertz to 30 exahertz (3·1016 Hz to 3·1019 Hz) and energies in the of range 100 eV to 100 keV .
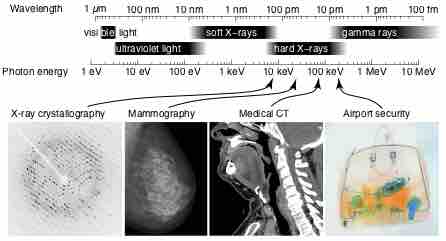
X-Ray Spectrum and Applications
X-rays are part of the electromagnetic spectrum, with wavelengths shorter than those of visible light. Different applications use different parts of the X-ray spectrum.
X-rays can be generated by an x-ray tube, a vacuum tube that uses high voltage to accelerate the electrons released by a hot cathode to a high velocity. The high-velocity electrons collide with a metal target, the anode, creating the x-rays. The maximum energy of the produced x-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 80-kV tube cannot create x-rays with an energy greater than 80 keV. When the electrons hit the target, x-rays are created through two different atomic processes:
- X-ray fluorescence, if the electron has enough energy that it can knock an orbital electron out of the inner electron shell of a metal atom. As a result, electrons from higher energy levels fill up the vacancy, and x-ray photons are emitted. This process produces an emission spectrum of x-rays at a few discrete frequencies, sometimes referred to as the spectral lines. The spectral lines generated depend on the target (anode) element used and therefore are called characteristic lines. Usually these are transitions from upper shells into the K shell (called K lines), or the L shell (called L lines), and so on.
- Bremsstrahlung, literally meaning braking radiation. Bremsstrahlung is radiation given off by the electrons as they are scattered by the strong electric field near the high-Z (proton number) nuclei. These x-rays have a continuous spectrum. The intensity of the x-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons, the voltage on the x-ray tube.
Both of these x-ray production processes are inefficient, with a production efficiency of only about one percent. Therefore, to produce a usable flux of x-rays, most of the electric power consumed by the tube is released as heat waste. The x-ray tube must be designed to dissipate this excess heat.
A specialized source of x-rays that is becoming widely used in research is synchrotron radiation, which is generated by particle accelerators. Its unique features are x-ray outputs many orders of magnitude greater than those of x-ray tubes, wide x-ray spectra, excellent collimation, and linear polarization.