Fluorescence and Phosphorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of photoluminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. However, when the absorbed electromagnetic radiation is intense, it is possible for one electron to absorb two photons; this two-photon absorption can lead to emission of radiation having a shorter wavelength than the absorbed radiation. The emitted radiation may also be of the same wavelength as the absorbed radiation, termed "resonance fluorescence".
Fluorescence occurs when an orbital electron of a molecule or atom relaxes to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy. The most striking examples of fluorescence occur when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, and the emitted light is in the visible region .

Fluorescence
Fluorescent minerals emit visible light when exposed to ultraviolet light
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. Excitation of electrons to a higher state is accompanied with the change of a spin state . Once in a different spin state, electrons cannot relax into the ground state quickly because the re-emission involves quantum mechanically forbidden energy state transitions. As these transitions occur very slowly in certain materials, absorbed radiation may be re-emitted at a lower intensity for up to several hours after the original excitation.
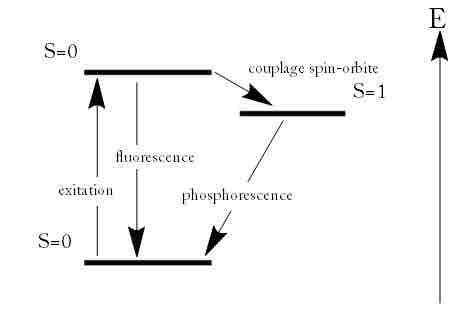
Fluorescence and Phosphorescence
Energy scheme used to explain the difference between fluorescence and phosphorescence
Commonly seen examples of phosphorescent materials are the glow-in-the-dark toys, paint, and clock dials that glow for some time after being charged with a bright light such as in any normal reading or room light . Typically the glowing then slowly fades out within minutes (or up to a few hours) in a dark room.

Phosphorescence
Phosphorescent material glowing in the dark.