Heat as Energy Transfer
Consider two objects at different temperatures that are brought together. Energy is transferred from the hotter object to the cooler one, until both objects reach thermal equilibrium (i.e., both become the same temperature). How is this energy transferred? No work is done by either object, because no force acts through a distance. The transfer of energy is caused by the temperature difference, and ceases once the temperatures are equal . This observation leads to the following definition of heat: Heat is the spontaneous transfer of energy due to a temperature difference .
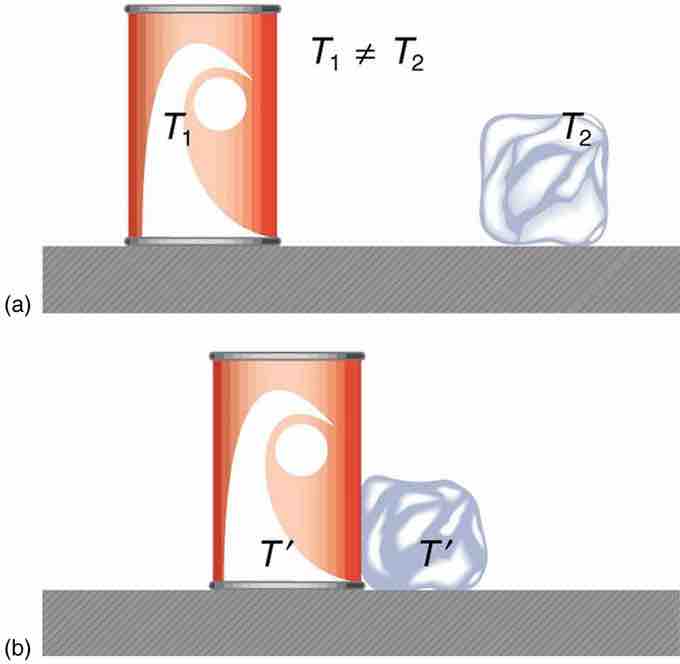
Heat Transfer and Equilibrium
(a) The soft drink and the ice have different temperatures, T1 and T2, and are not in thermal equilibrium. (b) When the soft drink and ice are allowed to interact, energy is transferred until they reach the same temperature T, achieving equilibrium. Heat transfer occurs due to the difference in temperatures. In fact, since the soft drink and ice are both in contact with the surrounding air and bench, the equilibrium temperature will be the same for both.
Heat is often confused with temperature. For example, we may say the heat was unbearable, when we actually mean that the temperature was high. Heat is a form of energy, whereas temperature is not. The misconception arises because we are sensitive to the flow of heat, rather than the temperature.
Units
Owing to the fact that heat is a form of energy, it has the SI unit of joule (J). The calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºC —specifically, between 14.5ºC and 15.5ºC, since there is a slight temperature dependence. Another common unit of heat is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by 1.00ºC. Since mass is often specified in kilograms, kilocalorie is commonly used. Food calories (given the notation Cal, and sometimes called "big calorie") are actually kilocalories (1kilocalorie=1000 calories), a fact not easily determined from package labeling in the United States, but more common in Europe and elsewhere. In some engineering fields, the British Thermal Unit (BTU), equal to about 1.055 kilo-joules, is widely used.
The total amount of energy transferred as heat is conventionally written as Q for algebraic purposes. Heat released by a system into its surroundings is by convention a negative quantity (Q < 0); when a system absorbs heat from its surroundings, it is positive (Q > 0).
Mechanical Equivalent of Heat
It is also possible to change the temperature of a substance by doing work. Work can transfer energy into or out of a system. This realization helped establish the fact that heat is a form of energy. James Prescott Joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat—the work needed to produce the same effects as heat transfer. In terms of the units used for these two terms, the best modern value for this equivalence is 1.000 kcal = 4186 J. We consider this equation as the conversion between two different units of energy.
Figure 1 shows one of Joule's most famous experimental setups for demonstrating the mechanical equivalent of heat. It demonstrated that work and heat can produce the same effects, and helped establish the principle of conservation of energy. Gravitational potential energy (PE) (work done by the gravitational force) is converted into kinetic energy (KE), and then randomized by viscosity and turbulence into increased average kinetic energy of atoms and molecules in the system, producing a temperature increase. His contributions to the field of thermodynamics were so significant that the SI unit of energy was named after him.
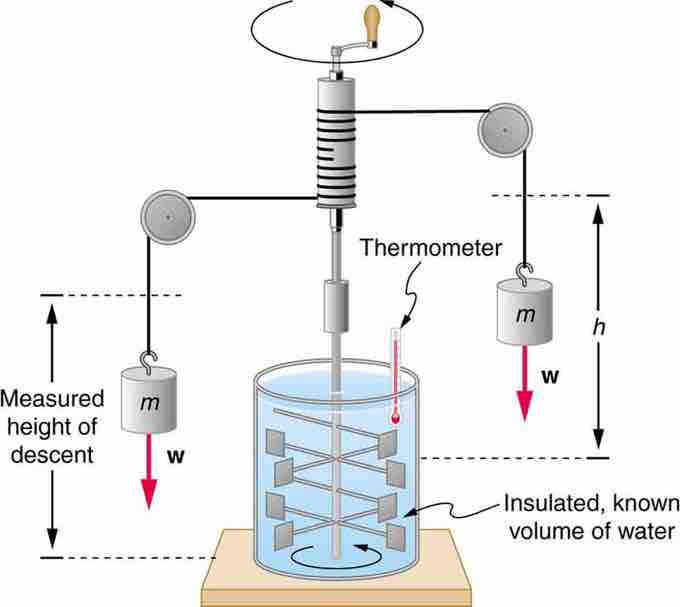
Figure 1 Equivalence of Heat and Work
Schematic depiction of Joule's experiment that established the equivalence of heat and work
Heat added or removed from a system changes its internal energy (a concept we will discuss in the following section) and thus its temperature. Such a temperature increase is observed while cooking. However, adding heat does not necessarily increase the temperature. An example is melting of ice; that is, when a substance changes from one phase to another. Work done on the system or by the system can also change the internal energy of the system. Joule demonstrated that the temperature of a system can be increased by stirring. If an ice cube is rubbed against a rough surface, work is done by the frictional force. A system has a well-defined internal energy, but we cannot say that it has a certain "heat content" or "work content." We use the phrase "heat transfer" to emphasize its nature.