Concept
Version 9
Created by Boundless
Heat as Energy Transfer
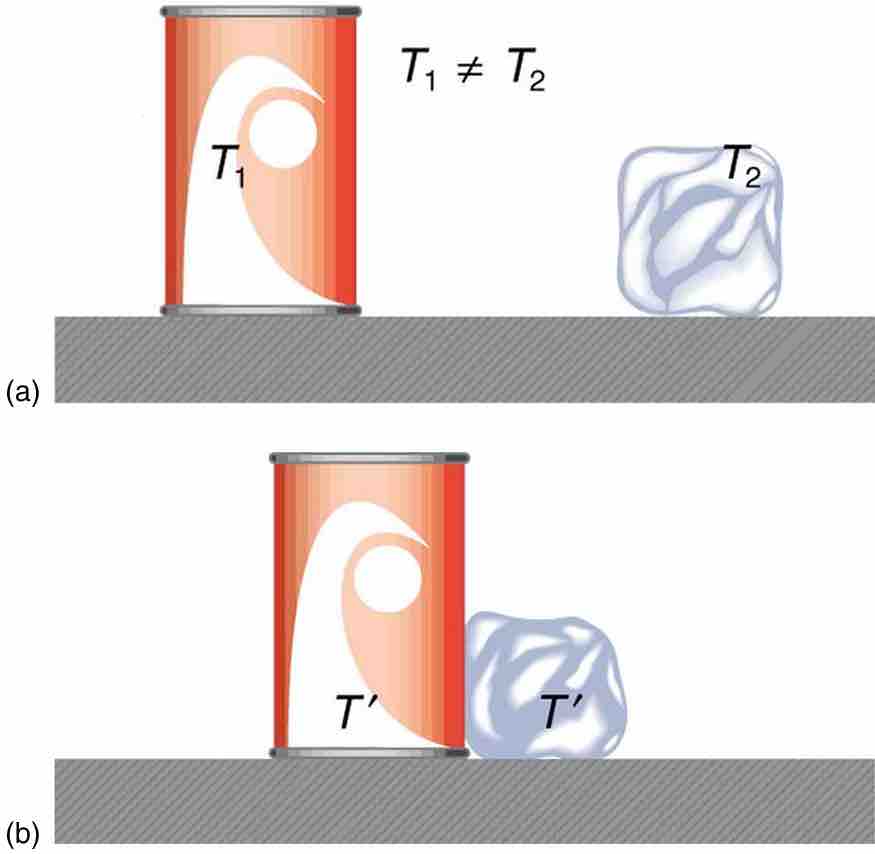
Heat Transfer and Equilibrium
(a) The soft drink and the ice have different temperatures, T1 and T2, and are not in thermal equilibrium. (b) When the soft drink and ice are allowed to interact, energy is transferred until they reach the same temperature T, achieving equilibrium. Heat transfer occurs due to the difference in temperatures. In fact, since the soft drink and ice are both in contact with the surrounding air and bench, the equilibrium temperature will be the same for both.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. October 13, 2012."
http://cnx.org/content/m42223/latest/?collection=col11406/1.7
OpenStax CNX
CC BY 3.0.