Atmospheric Pressure
An important distinction must be made as to the type of pressure quantity being used when dealing with pressure measurements and calculations. Atmospheric pressure is the magnitude of pressure in a system due to the atmosphere, such as the pressure exerted by air molecules (a static fluid) on the surface of the earth at a given elevation. In most measurements and calculations, the atmospheric pressure is considered to be constant at 1 atm or 101,325 Pa, which is the atmospheric pressure under standard conditions at sea level.
Atmospheric pressure is due to the force of the molecules in the atmosphere and is a case of hydrostatic pressure. Depending on the altitude relative to sea level, the actual atmospheric pressure will be less at higher altitudes and more at lower altitudes as the weight of air molecules in the immediate atmosphere changes, thus changing the effective atmospheric pressure. Atmospheric pressure is a measure of absolute pressure and can be affected by the temperature and air composition of the atmosphere but can generally be accurately approximated to be around standard atmospheric pressure of 101,325 Pa. Within the majority of earth's atmosphere, pressure varies with height according to . In this equation p0 is the pressure at sea level (101,325 Pa), g is the acceleration due to gravity, M is the mass of a single molecule of air, R is the universal gas constant, T0 is the standard temperature at sea level, and h is the height relative to sea level.
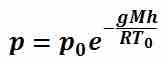
Pressure and Height
Atmospheric pressure depends on altitude or height.
Gauge Pressure
For most applications, particularly those involving pressure measurements, it is more practical to use gauge pressure than absolute pressure as a unit of measurement. Gauge pressure is a relative pressure measurement which measures pressure relative to atmospheric pressure and is defined as the absolute pressure minus the atmospheric pressure. Most pressure measuring equipment give the pressure of a system in terms of gauge pressure as opposed to absolute pressure. For example, tire pressure and blood pressure are gauge pressures by convention, while atmospheric pressures, deep vacuum pressures, and altimeter pressures must be absolute.
For most working fluids where a fluid exists in a closed system, gauge pressure measurement prevails. Pressure instruments connected to the system will indicate pressures relative to the current atmospheric pressure. The situation changes when extreme vacuum pressures are measured; absolute pressures are typically used instead.
To find the absolute pressure of a system, the atmospheric pressure must then be added to the gauge pressure. While gauge pressure is very useful in practical pressure measurements, most calculations involving pressure, such as the ideal gas law, require pressure values in terms of absolute pressures and thus require gauge pressures to be converted to absolute pressures.