Section 2
Entropy
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
Microstates and Entropy
Energy can be shared between microstates of a system. With more available microstates, the entropy of a system increases.

Changes in Energy
The concept of entropy can be described qualitatively as a measure of energy dispersal at a specific temperature.
Standard Entropy
The standard entropy of a substance (its entropy at 1 atmospheric pressure) helps determine if a reaction will take place spontaneously.
Changes in the Entropy of Surroundings
Irreversible reactions result in a change in entropy to the surroundings.
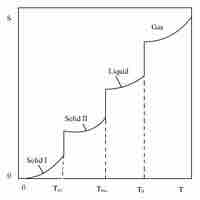
The Third Law of Thermodynamics and Absolute Energy
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.