Solid Solubility and Temperature
The solubility of a given solute in a given solvent typically depends on temperature. Many salts show a large increase in solubility with temperature. Some solutes exhibit solubility that is fairly independent of temperature. A few, such as cerium(III) sulfate, become less soluble in water as temperature increases. This temperature dependence is sometimes referred to as retrograde or inverse solubility, and exists when a salt's dissolution is exothermic; this can be explained because, according to Le Chatelier's principle, extra heat will cause the equilibrium for an exothermic process to shift towards the reactants.
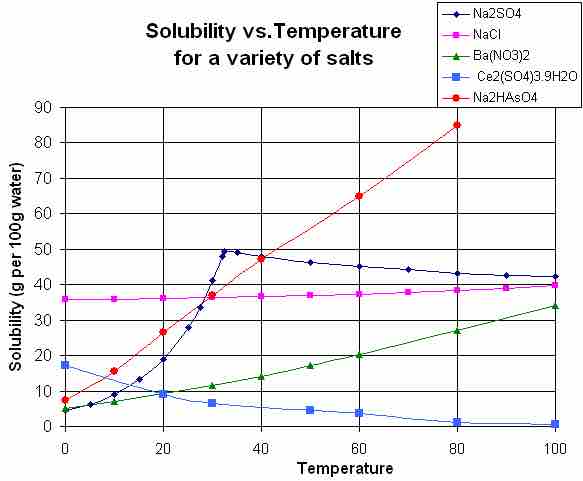
Solubility Versus Temperature
This chart shows the solubility of various substances in water at a variety of temperatures (in degrees Celsius). Notice how NaCl's solubility is relatively constant regardless of temperature, whereas Na2SO4's solubility increases exponentially over 0–35 degrees Celsius and then abruptly begins to decrease.
Theoretical Perspective
As the temperature of a solution is increased, the average kinetic energy of the molecules that make up the solution also increases. This increase in kinetic energy allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
The average kinetic energy of the solute molecules also increases with temperature, and it destabilizes the solid state. The increased vibration (kinetic energy) of the solute molecules causes them to be less able to hold together, and thus they dissolve more readily.
Application in Recrystallization
A useful application of solubility is recrystallizaton. During recrystallization, an impure substance is taken up in a volume of solvent at a temperature at which it is insoluble, which is then heated until it becomes soluble. The impurities dissolve as well, but when the solution is cooled, it is often possible to selectively crystallize, or precipitate, the desired substance in a purer form.