In organic chemistry, an addition reaction is, in its simplest terms, an organic reaction in which two or more molecules combine to form a larger molecule. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon-carbon double bonds (alkenes) or with triple bonds (alkynes). Molecules containing carbon based double bonds, like carbonyl (C=O) groups or imine (C=N) groups, can also undergo addition. An addition reaction is the opposite of an elimination reaction. For instance, an alkene's hydration reaction adds water to an alkene, and an alcohol's dehydration removes water from the alkene; these two reactions are opposites and are considered addition-elimination pairs.
There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions also exist: free radical addition and cycloadditions. In the related addition-elimination reaction, an addition reaction is followed by an elimination reaction; in most reactions, this involves addition to carbonyl compounds in nucleophilic acyl substitution. The hydrolysis of nitriles to carboxylic acids is also a form of addition-elimination.
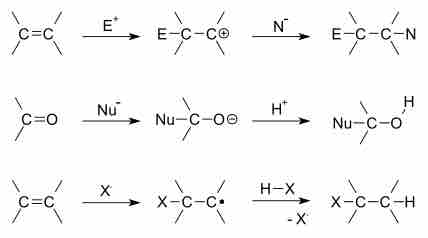
General outline of addition reactions
Top to bottom: electrophilic addition to alkene, nucleophilic addition of nucleophile to carbonyl, and free radical addition of halide to alkene.
Electrophilic Addition
Most addition reactions to alkenes follow the mechanism of electrophilic addition. An example is the Prins reaction, where the electrophile is a carbonyl group. In halogenation, adding elementary bromine or chlorine to alkenes yields dibromo- and dichloro-alkanes, respectively.
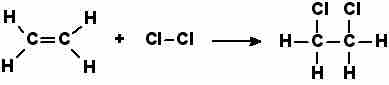
Adding chlorine to ethene
This shows an addition reaction, where chlorine and ethene form single molecule 1,2-dichloroethane.
Nucleophilic Addition Reactions
In nucleophilic addition reactions, the nucleophile donates an electron pair to the electrophile (one of the atoms in the double bond). In hydrohalogenation, the nucleophile is the halogen. The halogen donates an electron pair to one of the carbons participating in a double bond. The hydrogen is then added to the other carbon that was originally double bonded. Addition of hydrohalic acids like HCl or HBr to alkenes yields the corresponding haloalkanes. An example of this type of reaction is:
Free Radical Additions of Halogens to Alkanes
In free radical additions of halogens to alkanes (or alkenes), a radical halogen can attack an alkane to produce another radical, in this case a radical version of the alkane. The radical alkane can attack another compound, producing another radical that can continue on to attack another compound.
Polymerization of alkenes is an economically important reaction that yields polymers of high industrial value, such as the plastics polyethylene and polypropylene. Polymerization can either proceed via a free-radical or an ionic mechanism.