Concept
Version 9
Created by Boundless
Periods 1 through 3
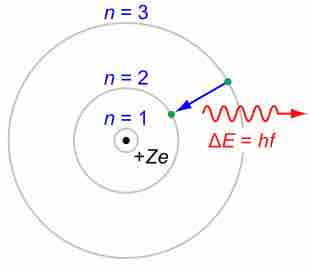
A simplified depiction of atomic structure
With the nucleus at the center with a positive charge, the electrons are "organized" in energy levels, or shells, at increasing distances from the nucleus. The distance between the n = 2 and n = 3 shells in this illustration is the difference in energy between them.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: