The modern periodic table organizes the known elements in several ways: it lists them in order of patterns of atomic weight, electron configuration, reactivity, and electronegativity. It is such a good method of organizing and presenting the known elements that it has been used to successfully predict the existence of certain elements. Today, it is applied not only by chemists but also in all related sciences to understand the properties and reactivity of atoms and molecules. The table has recognizable origins in the 17th century and draws on knowledge and experience of medieval and earlier eras.
History of the Periodic Table
Atomic theory dates back to the ancient Greek philosophers and those of Hellenistic Egypt. They theorized that all substances were made of fundamental building blocks; however, the nature of those blocks was the object of fierce debate.
The fundamental blocks were called atoms, derived from the Greek "atmos," meaning "indivisible." Early atomic theory attempted to explain properties of matter by assigning attributes to atoms that might match the attributes of the various matter they combined to form, such as slipperiness, liquidity, color, and cohesiveness. Philosophers categorized the world around them by property and function, a type of approach that later led to the development of the periodic table of elements.
In the Middle Ages, practitioners of alchemy sought to make gold and silver from lead. Although their efforts were in vain, their investigation has ultimately led to a systematic understanding of the chemical world. It also established the mindset that gave us the periodic table of elements.
Alchemists were influenced by international trade, especially along the Silk Road between China and Europe. Chemical knowledge spread across cultures, and by about the middle of the 18th century, there were already 33 known elements. At the beginning of the 19th century, Joseph Proust and others were demonstrating the Law of Definite Proportions experimentally. This provided fundamental evidence that matter existed in pure compounds as opposed to just mixtures of any proportion. These observations strengthened the atomic theory and demanded a systematic method of organizing the elements.
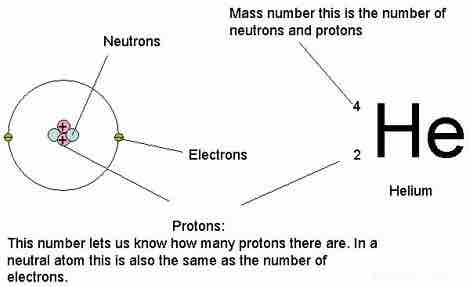
Notation in the periodic table
The notation in the periodic table includes references to atomic mass and atomic number.
The Modern View of the Periodic Table
Scientists began to notice similarities and patterns among known elements, and a great research interest of the 19th century was to develop a systematic method to report and classify them. Russian chemistry professor Dmitri Mendeleev and German chemist Julius Meyer independently presented their own versions of the periodic table in 1869 and 1870. Mendeleev's approach was ultimately adopted for several reasons: For one, he left gaps for elements that had yet to be discovered. In doing so, he predicted the elements gallium and germanium. He also placed atoms based principally on their chemical properties, not atomic mass. As it turns out, organizing by chemical family correctly sorts most of the elements by their atomic number; atomic mass is not perfectly correlated with atomic number.

The periodic table of the elements
A modern representation of the periodic table showing organization by atomic number and valence. Note the atomic masses are not included in this particular periodic table, however they are typically included below the element symbol.
The modern version of Mendeleev's periodic table now contains some 118 different elements. In the periodic table, the number above the element's symbol is the atomic number, which represents the number of protons in the nucleus. The atomic mass is given by the sum of the neutrons and protons.