Section 3
Periodic Trends
By Boundless
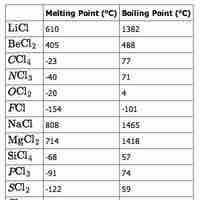
The physical properties of elements vary across a period, mostly as a function of bonding.

The physical properties (notably, melting and boiling points) of the elements in a given group vary as you move down the table.
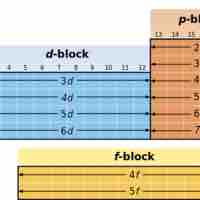
The electron configuration of a given element can be predicted based on its location in the periodic table.
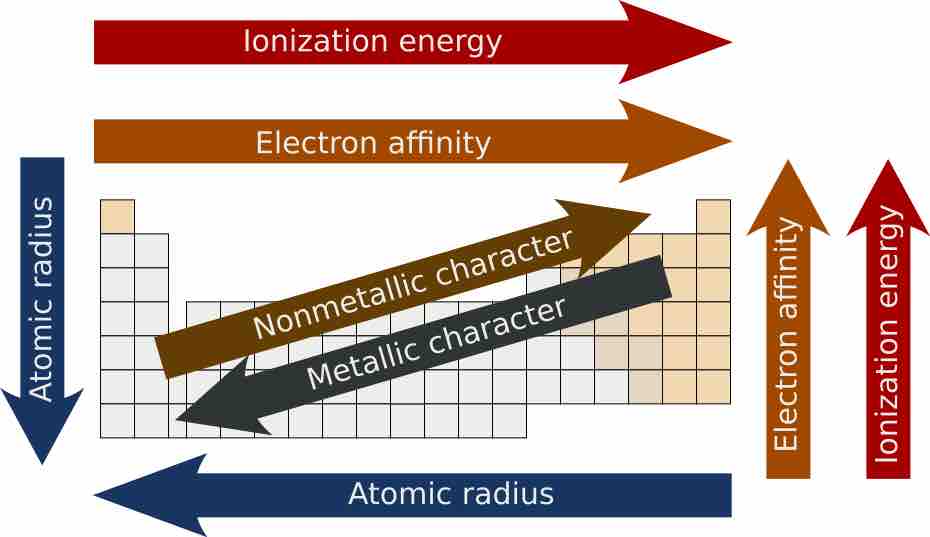
Atomic radii decrease from left to right across a period and increase from top to bottom along a group.

Similarly charged ions tend to decrease in size across a period (row) and increase in size down a group (column).

The ionization energy tends to increase as one moves from left to right across a given period or up a group in the periodic table.

The electron affinity of the elements generally increases across a period and sometimes decreases down a group in the periodic table.