Concept
Version 12
Created by Boundless
General Rules for Assigning Electrons to Atomic Orbitals
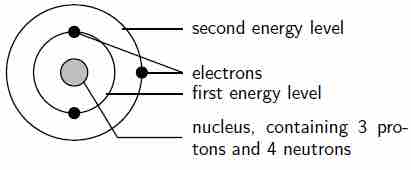
The arrangement of electrons in a lithium atom
Lithium (Li) has an atomic number of 3, meaning that in a neutral atom, the number of electrons will be 3. The energy levels are shown as concentric circles around the central nucleus, and the electrons are placed from the inside out. The first two electrons are found in the first energy level, and the third electron is found in the second energy level.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Free High School Science Texts Project, The Atom: Energy Quantisation and Electron Configuration. October 15, 2012."
http://cnx.org/content/m39967/latest/
OpenStax CNX
CC BY 3.0.