A carboxyl group (COOH) is a functional group consisting of a carbonyl group (C=O) with a hydroxyl group (O-H) attached to the same carbon atom. Carboxyl groups have the formula -C(=O)OH, usually written as -COOH or CO2H. Carboxylic acids are a class of molecules which are characterized by the presence of one carboxyl group. As proton donors, carboxylic acids are characterized as Brønsted-Lowry acids. Acids with two or more carboxylic groups are called dicarboxylic, tricarboxylic, etc. Salts and esters of carboxylic acids are called carboxylates. Carboxylate ions are resonance-stabilized. This increased stability leads to increased acidity compared to that of alcohols. Generally, in IUPAC nomenclature, carboxylic acids have an "-oic acid" suffix, although "-ic acid" is the suffix most commonly used.
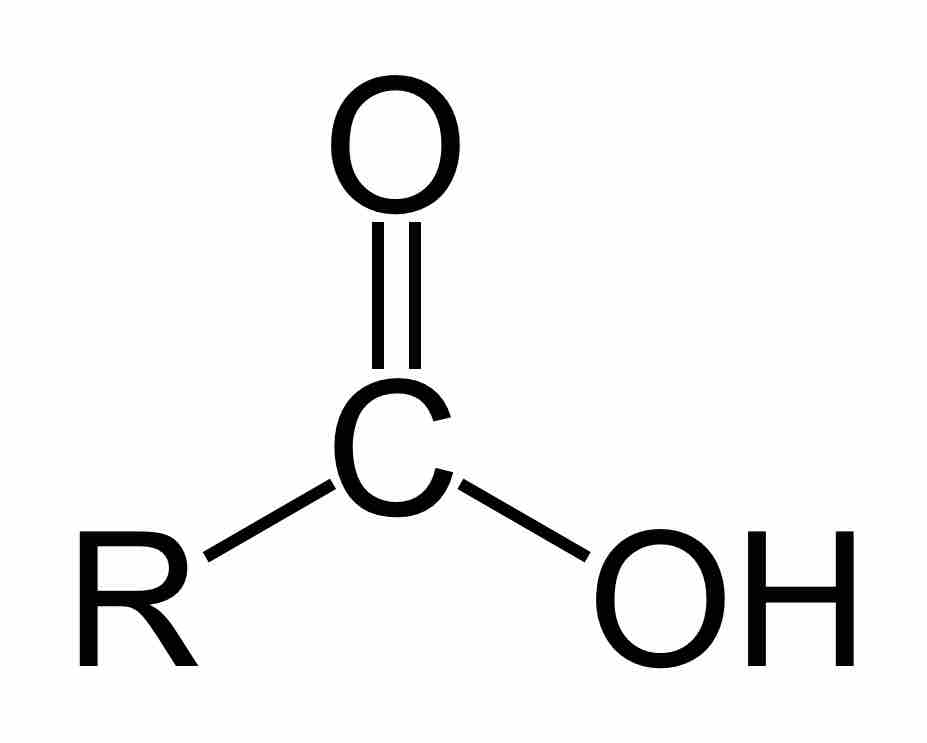
A carboxylic acid
Carboxylic acids are organic oxoacids characterized by the presence of at least one carboxyl group, which has the formula -C(=O)OH, usually written as -COOH or -CO2H.
Physical Properties of Carboxylic Acids
Carboxylic acids act as both hydrogen bond acceptors, due to the carbonyl group, and hydrogen bond donors, due to the hydroxyl group. As a result, they often participate in hydrogen bonding. Carboxylic acids usually exist as dimeric pairs in nonpolar media because of their tendency to "self-associate." This tendency to hydrogen bond gives them increased stability as well as higher boiling points relative to the acid in aqueous solution. Carboxylic acids are polar molecules; they tend to be soluble in water, but as the alkyl chain gets longer, their solubility decreases due to the increasing hydrophobic nature of the carbon chain. Carboxylic acids are characterized as weak acids, meaning that they do not fully dissociate to produce H+ cations in a neutral aqueous solution.
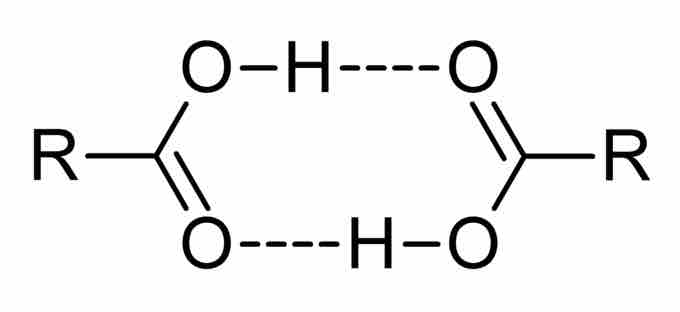
Hydrogen bonding between carboxylic acids
Carboxylic acids hydrogen bond with themselves, giving them an increased level of stability.
Spectroscopy of Carboxylic Acids
Carboxylic acids can be characterized by IR spectroscopy; they exhibit a sharp band associated with vibration of the C-O bond between 1680 and 1725 cm-1. Additionally, a broad peak appears in the 2500 to 3000 cm-1 region. By 1H NMR spectroscopy, the hydroxyl hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.
Applications and Reactivity of Carboxylic Acids
Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, and food additives. As such, they are often produced industrially on a large scale. Carboxylic acids are generally produced from oxidation of aldehydes and hydrocarbons, and base catalyzed dehydrogenation of alcohols. They can be produced in the laboratory for small scale reactions via the oxidation of primary alcohols or aldehydes, oxidative cleavage of olefins, and through the hydrolysis of nitriles, esters, or amides.
Carboxylic acids are widely used as precursors to produce other compounds. Upon exposure to a base, the carboxylic acid is deprotonated and forms a carboxylate salt. They also react with alcohols to produce esters and can undergo reduction reactions by hydrogenation or the use of reducing agents. There are also various specialized reactions that carboxylic acids participate in that lead to the formation of amines, aldehydes, and ketones.