The benzene ring is frequently noted for the stability it gains from its aromaticity. However, aromatic compounds can participate in a variety of chemical reactions, including a range of substitution, coupling, and hydrogenation reactions. The electrons in the pi system of the benzene ring are responsible for the reactivity observed. While aromatic compounds are best represented by a continuous electron density evenly distributed around the aromatic core, the alternating single and double bonds that are commonly drawn are very useful when predicting the reactivity of aromatic compounds. Many reactions common to alkenes (carbon-carbon double bonds) also function in a similar fashion with the "double bonds" in aromatic compounds, though generally the activation barrier is higher due to the stabilizing force of aromaticity (ca. 36 kcal/mol).
Aromatic Substitution
An example of an aromatic substitution reaction is shown below. In the presence of strong sulfuric and nitric acid, a nitro group can be added to the ring.
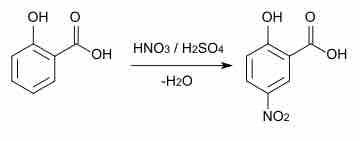
Aromatic substitution
Example of an aromatic substitution reaction. The double bond attacks the NO2 cation, then a proton (hydrogen cation) is lost to rearomatize the system.
Nucleophilic Aromatic Substitutions
In a nucleophilic aromatic substitution reaction, a nucleophile displaces a substituent on an aromatic ring. The replaced species is typically a good leaving group, like nitrogen gas or a halide ion. The presence of an electron-withdrawing group on the ring can speed up the progress of this class of reactions. Chemically, this is similar to an addition reaction to a Michael acceptor or other electron-deficient, unsaturated system, followed by an elimination reaction.
Electrophilic Aromatic Substitutions
In an electrophilic aromatic substitution reaction, a substituent on an aromatic ring is displaced by an electrophile. These reactions include aromatic nitration, aromatic halogenation, aromatic sulfonation, and Friedel-Crafts acylations and alkylations. These reactions can involve a resonance-stabilized carbocation intermediate known as a sigma complex. The reactivity can be thought of in terms of an alkene attacking a cationic species, such as in the first step of an acid-catalyzed hydration of an alkene.
A number of patterns have been observed regarding the reaction of substituted benzene rings. These observations have been generalized to provide a predictive rule for electrophilic aromatic substitutions. It states that an electron-donating substituent generally accelerates substitution and directs reactivity toward the positions that are ortho and para to it on the ring, while an electron-withdrawing substituent will slow reaction progress and favor the meta position on the ring.
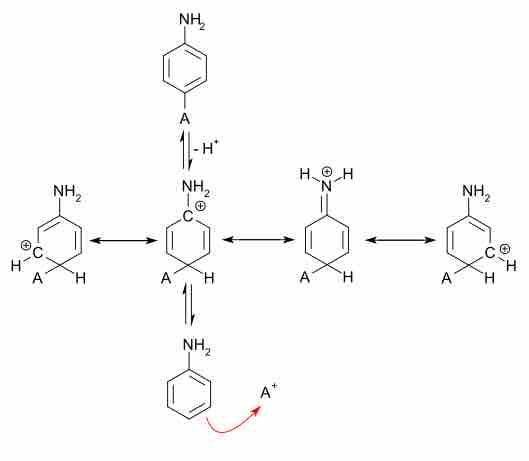
Electrophilic aromatic substitution (EAS)
This reaction mechanism takes place from bottom to top. EAS occurs ortho or para to electron donating groups, such as amines, due to the stabilization of the intermediate positive charge. The four structures drawn in the middle of the diagram are all resonance structures. Due to the electrons provided by the NH2 group, this intermediate is stabilized, and the para-substitution is favored. As an exercise, draw out the stabilization of the positive charge when ortho substitution occurs.
Coupling Reactions
Coupling reactions are reactions involving a metal catalyst that can result in the formation of a carbon-carbon bond between two radicals.
Hydrogenation
Hydrogenation can be used to create a fully saturated ring system. This is similar to the hydrogenation of an alkene to form an alkane, albeit more difficult due to the stability of the aromatic system.