Alkene Structures
Alkenes contain a double bond that is composed of one sigma and one pi bond between two carbon atoms. The sigma bond has similar properties to those found in alkanes, while the pi bond is more reactive. The carbon atoms in the double bond are sp2 hybridized, forming a planar structure. Rotation around the double bond is disfavored, so alkenes form fairly stable isomers depending on the positioning of substituents on the same (cis) or opposite (trans) sides of the double bond. These isomers are called diastereoisomers.
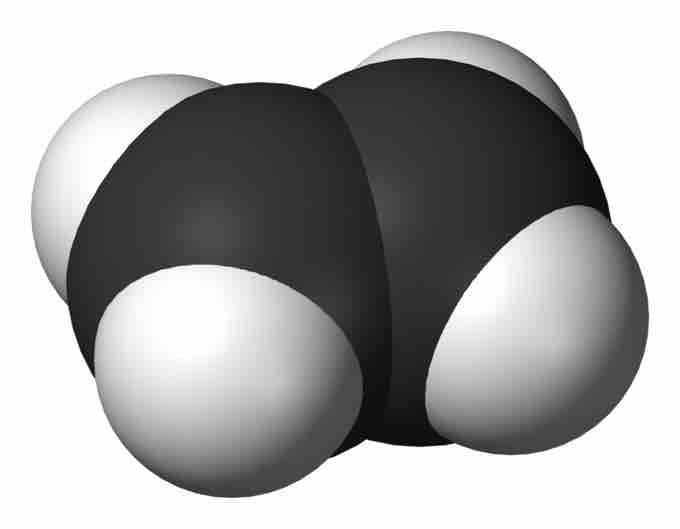
Ethylene
A space-filling model of ethylene, the simplest alkene, showing its planar structure.
Physical Properties of Alkenes
The melting and boiling points of alkenes are determined by the regularity of the packing, or the closeness, of these molecules. Alkene isomers that can achieve more regular packing have higher melting and boiling points than molecules with the same molecular formula but weaker dispersion forces. Alkenes are non-polar, and they are both immiscible in water and less dense than water. They are generally soluble in organic solvents. In addition, they do not conduct electricity.
Reactivity of Alkenes
Alkenes are more reactive than their related alkanes due to the relative instability of the double bond. They are more likely to participate in a variety of reactions, including combustion, addition, hydrogenation, and halogenation reactions. Alkenes can also be reacted, typically in the presence of a catalyst, to form polymers.
Applications
Large amounts of ethylene are produced from natural gas via thermal cracking. It is an important raw material for the synthesis of a number of plastics.
Thermal cracking
The factory of the Shukhov cracking process by the great Russian engineer and scientist Vladimir Shukhov (1853-1939) in 1934. In petroleum geology and chemistry, thermal cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors.