Aluminosilicate minerals are composed of aluminum, silicon, oxygen, and countercations. They are a major component of kaolin and other clay minerals.
Andalusite, Kyanite, and Sillimanite

Andalusite
Andalusite is an aluminium nesosilicate mineral with the chemical formula Al2SiO5.
Andalusite, kyanite, and sillimanite are all naturally occurring aluminosilicate minerals that have the composition Al2SiO5 . The triple point of these three polymorphs is located at a temperature of 500 °C and a pressure of 0.4 GPa. These three minerals are commonly used as index minerals in metamorphic rocks. Each of these minerals occurs under different temperature-pressure regimes, and they are therefore rarely found together in the same rock. Because of this, the three minerals are a useful tool in identifying the pressure-temperature paths of the host rock in which they are found.
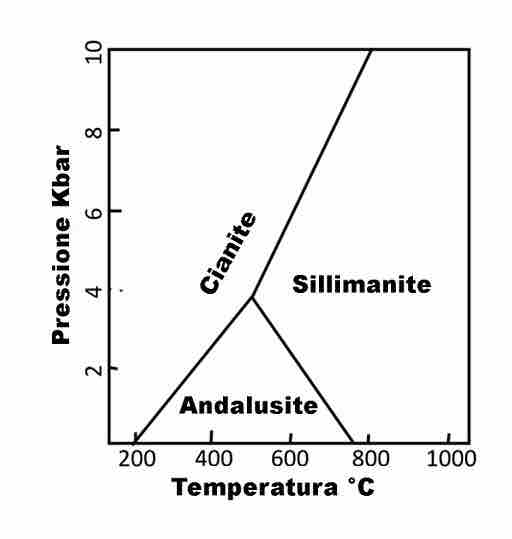
Phase Diagram of Aluminosilicate Mineral
The phase diagram of Al2SiO5 showing its different forms (called "polymorphs"). (Note: What is referred to as "Cianite" in the figure corresponds to "kyanite" in the text.)
Zeolites
Hydrated aluminosilicate minerals are referred to as zeolites. They have porous structures and are naturally occurring materials . Zeolites are commonly used as commercial absorbents. Zeolites are used for a variety of tasks, including purifying water, catalyzing reactions, preparing certain advanced materials, and nuclear reprocessing. They are used to extract nitrogen from air, which then increases the general oxygen content for various industrial and medical purposes. They are used most frequently in the production of laundry detergents, but are also used in medicine and in agriculture.

Zeolite
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents.
Calcium Aluminosilicates
Calcium aluminosilicate, an aluminosilicate compound with calcium cations, most typically has the chemical formula, CaAl2Si2O8. As a food additive, it is sometimes designated "E556". In minerals, as in feldspar, it can be found as anorthite, an end-member of the plagioclase series.
Sodium Aluminosilicates
Sodium aluminosilicates are acidic salts that is composed of sodium, aluminum, silicon and oxygen. These can be found as synthetic, amorphous, sodium aluminosilicates, a few naturally-occurring minerals, and synthetic zeolites. Synthetic, amorphous, sodium aluminosilicate is widely used as a food additive, E-554.