The Chemistry of Phosphorus Compounds
The chemistry of phosphorus is often dominated by the strength of the oxygen-phosphorus bond, which is around 152 kcal/mol (kilocalories per mole). This extremely strong bond is the driving force behind several reactions involving phosphorus. For example, the reaction of PCl5 with water to become H3PO4 allows it to serve as a drying agent, or dessicant, with the P-O bond formation as the driving force. The oxygen-phosphorus bond also prohibits phosphorus from being observed in its elemental state in nature. It is always found as an oxide.
The majority of phosphorus-containing compounds are produced for use as fertilizers. For this purpose, phosphate-containing minerals are converted to phosphoric acid. Two distinct routes are employed; the main one is treatment of phosphate minerals with sulfuric acid. The other process utilizes white phosphorus, which may be produced by reaction and distillation from very low-grade phosphate sources. The white phosphorus is then oxidized to phosphoric acid and finally neutralized with a base to yield phosphate salts. Phosphoric acid obtained from white phosphorus is relatively pure and is the main source of phosphates used in detergents and other non-fertilizer applications.
Biological Significance
Inorganic phosphorus in the form of the phosphate PO43− is required for all known forms of life. It plays a major role in biological molecules; for example, it forms part of the structural framework of DNA and RNA. As such, phosphate salts are used as fertilizers to aid plant growth. Living cells also use phosphate to transport cellular energy in the form of adenosine triphosphate (ATP). Nearly every cellular process that uses energy obtains it in the form of ATP. ATP is also important for phosphorylation, a key regulatory and signal-transducing event in cells. Phospholipids are the main structural components of all cellular membranes and consist of a long alkyl chain terminating in a phosphate group. Calcium phosphate salts help to harden bones.
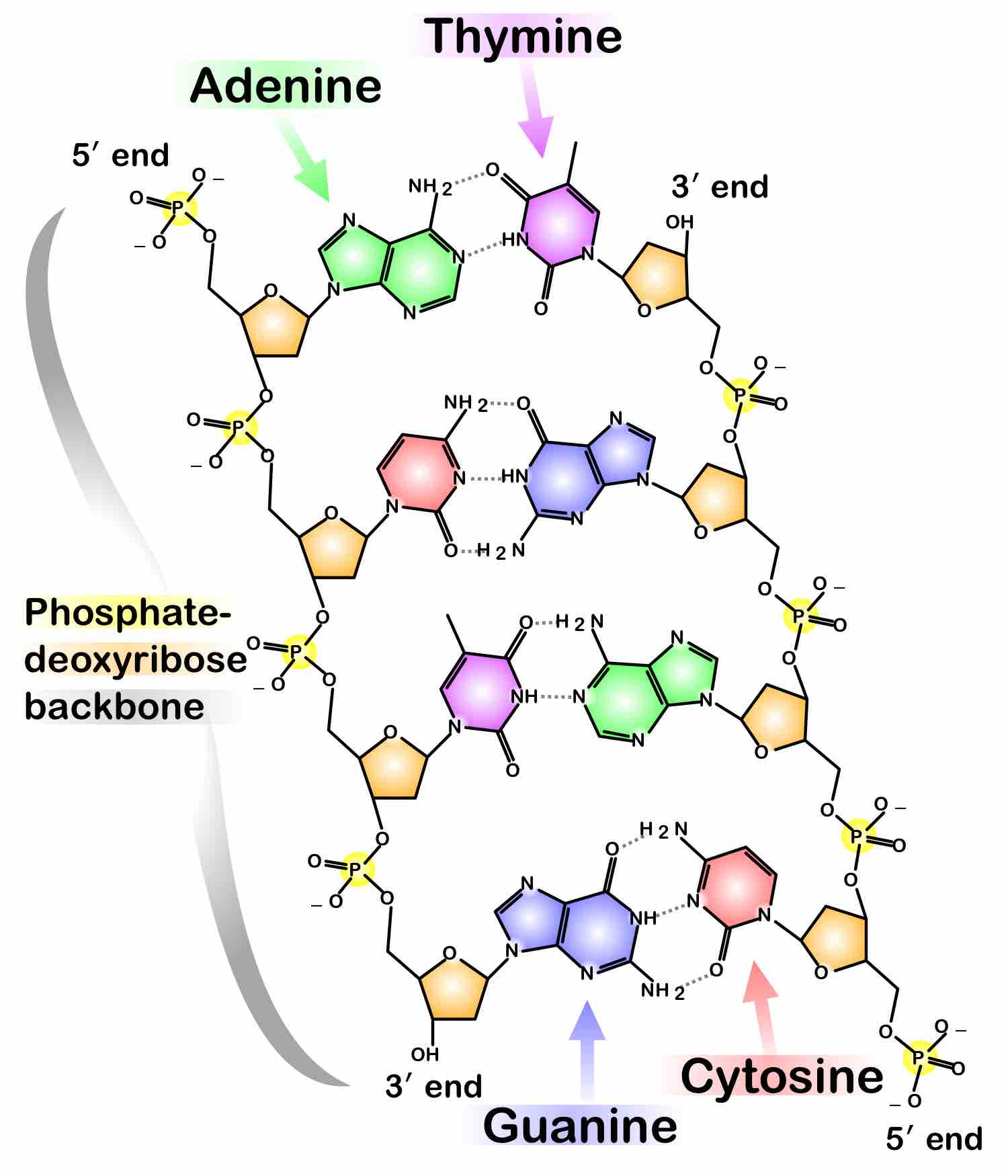
Phosphate in DNA
DNA strands have a phosphate-deoxyribose backbone. Two DNA strands are shown in the figure.
Living cells are defined by a membrane that separates it from its surroundings. Biological membranes are made from a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from glycerol, such that two of the glycerol hydroxyl (OH) groups have been replaced with fatty acids to form ester linkages, and the third hydroxyl group has been replaced with a phosphate group.
Oxoacids of Phosphorus
Phosphorous oxoacids are extensive, often commercially important, and sometimes structurally complicated. They all have acidic protons bound to oxygen atoms, and some have nonacidic protons that are bonded directly to phosphorus. Although many oxoacids of phosphorus are formed, only nine are important, and three are crucial: hypophosphorous acid, phosphorous acid, and phosphoric acid.
Phosphorus with an oxidation state of +1:
- Hypophosphorous acid, H3PO2, contains one acidic OH bond and two (relatively) non-acidic PH bonds.
Phosphorus with an oxidation state of +3:
- Phosphorous acid, H3PO3, contains two acidic OH bonds and one PH bond.
- Orthophosphorous acid, also written H3PO3, contains three acidic OH bonds and no PH bonds.
Phosphorus with an oxidation state of +5:
- Orthophosphoric acid, H3PO4, is the parent acid and most common oxidation state of phosphorus, with three acidic OH protons . Condensation between two phosphoric acid groups can lead to polyphosphates, such as meta- and polyphosphoric acid.
- Metaphosphoric acid, (HPO3)n, which occurs when phorphoric-acid molecules become bound together in ring structures, each vertex of the ring containing one acidic OH proton.
- Polyphosphoric acid, H(HPO3)nOH, which consists of multiple orthophosphoric acids bound together, each via a common oxygen.
Organophosphorus Compounds
Compounds with P-C and P-O-C bonds are often classified as organophosphorus compounds. They are widely used commercially. The PCl3 serves as a source of P+3 in routes to organophosphorus (III) compounds. For example, it is the precursor to triphenylphosphine:
Treatment of phosphorus trihalides with alcohols and phenols yields phosphites, such as triphenylphosphite:
Similar reactions occur for phosphorus oxychloride, yielding triphenylphosphate:
The Phosphate Group
There are several other phosphorus (V) compounds. The most prevalent compounds of phosphorus are derivatives of phosphate (PO43-), a tetrahedral anion. Phosphate is the conjugate base of phosphoric acid, which is produced on a massive scale for use in fertilizers. Since it is triprotic, phosphoric acid converts stepwise to three conjugate bases:
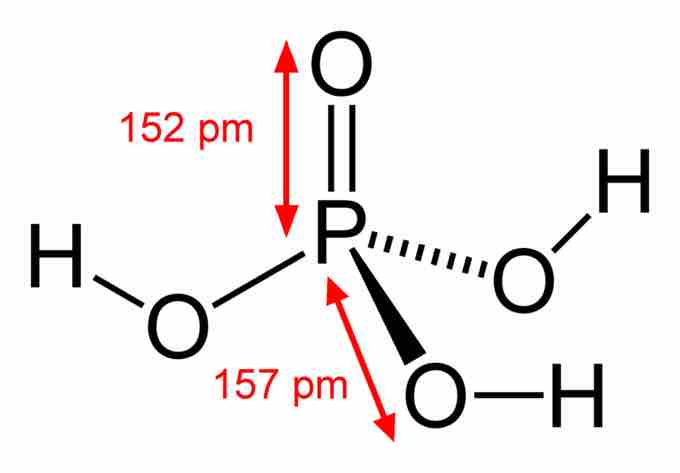
Phosphoric Acid
Phosphoric acid contains one P=O double bond and three P-O single bonds terminating in acidic OH groups.