Carbon is the chemical element with the symbol C and atomic number 6 (contains 6 protons in its nucleus). As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. The most common isotope of carbon has 6 protons and 6 neutrons, and has an atomic mass of 12.0107 amu. Its ground state electron configuration is 1s22s22p2. Its oxidation state ranges from 4 to -4, and it has an electronegativity value of 2.55 on the Pauling scale. It is a solid, and sublimes at 3,642 °C (it has the highest sublimation point of all the elements).
Carbon Allotropes
Carbon has several allotropes, or different forms in which it exists. Interestingly, carbon allotropes span a wide range of physical properties: diamond is the hardest naturally occurring substance, and graphite is one of the softest known substances. Diamond is transparent, the ultimate abrasive, and can be an electrical insulator and thermal conductor. Conversely, graphite is opaque, a very good lubricant, a good conductor of electricity, and a thermal insulator. Allotropes of carbon are not limited to diamond and graphite, but also include buckyballs (fullerenes), amorphous carbon, glassy carbon, carbon nanofoam, nanotubes, and others.
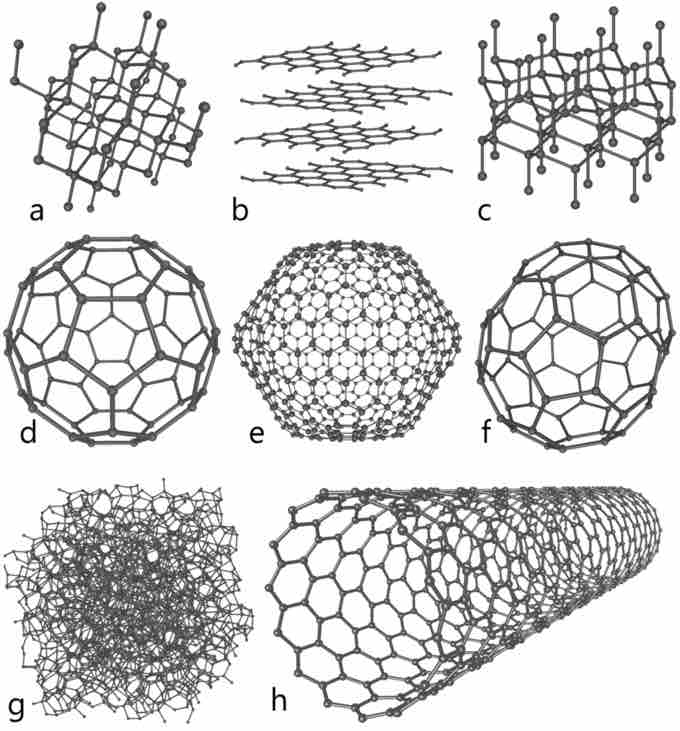
Allotropes of Carbon
Some allotropes of carbon: a) diamond, b) graphite, c) lonsdaleite, d–f) fullerenes (C60, C540, C70); g) amorphous carbon, h) carbon nanotube.
Chemical Reactivity of Carbon
Carbon compounds form the basis of all known life on Earth, and the carbon-nitrogen cycle provides some energy produced by the sun and other stars. Carbon has an affinity for bonding with other small atoms, including other carbon atoms, via the formation of stable, covalent bonds. Despite the fact that it is present in a vast number of compounds, carbon is weakly reactive compared to other elements under normal conditions. At standard temperature and pressure, it resists oxidation; it does not react with sulfuric acid, hydrochloric acid, chlorine, or any alkali metals. At higher temperatures, carbon will react with oxygen to give carbon oxides, and metals to give metal carbides.
Carbon has the ability to form very long chains of strong and stable interconnecting C-C bonds. This property allows carbon to form an almost infinite number of compounds; in fact, there are more known carbon-containing compounds than all the compounds of the other chemical elements combined, except those of hydrogen (because almost all organic compounds contain hydrogen as well).
Carbon Isotopes
Carbon has two stable, naturally occurring isotopes: carbon-12 and carbon-13. Carbon-12 makes 98.93% and carbon-13 forms the remaining 1.07%. The concentration of 12C is further increased in biological materials because biochemical reactions discriminate against 13C. Identification of carbon in NMR experiments is done with the isotope 13C. 14C is a radioactive isotope of carbon with a half-life of 5730 years. It has a very low natural abundance (0.0000000001%), and decays to 14N through beta decay. It is used in radiometric dating to determine the age of carbonaceous samples (of physical or biological origin) up to about 60,000 years old.
In total, there are 15 known isotopes of carbon and the shortest-lived of these is 8C, which decays through proton emission and alpha decay, and has a half-life of 1.98739 x 10−21 seconds. The exotic 19C exhibits a nuclear halo, which means its radius is appreciably larger than would be expected if the nucleus were a sphere of constant density.