Boron is a chemical element with the chemical symbol B and atomic number 5. Boron is produced entirely by cosmic ray spallation (as a result of nuclear reactions), and not by stellar nucleosynthesis (not within stars as a result of fusion or supernovae). As a result, it is a low-abundance element in both the solar system and the Earth's crust. Natural boron is composed of two stable isotopes: 11B is more abundant than 10B, which has a number of uses as a neutron-capturing agent. Several allotropes of boron exist. Amorphous boron is a brown powder, while crystalline boron is black, extremely hard (about 9.5 on the Mohs scale), and a poor conductor at room temperature. Elemental boron is used as a dopant in the semiconductor industry.
Chemical Properties of Boron
Elemental boron is rare and poorly studied because the material is extremely difficult to prepare. Most studies on "boron" involve samples that contain small amounts of carbon. Chemically, boron behaves more similarly to silicon than to aluminum. Crystalline boron is chemically inert and resistant to attack from boiling hydrofluoric or hydrochloric acid. When finely divided, it can be attacked slowly by hot, concentrated hydrogen peroxide or nitric acid; hot sulfuric acid; or a hot mixture of sulfuric and chromic acids.
On Earth, boron is concentrated by the water-solubility of its more common naturally-occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. Chemically uncombined boron, which is classified as a metalloid, is not found naturally on Earth. Industrially, very pure boron can be produced, but this is difficult, because boron tends to form refractory materials that contain small amounts of carbon or other elements.

Boron
Boron has a black-brown appearance.
Biological Significance of Boron
Boron is essential to life, although its exact physiological role in animals is not well-established. Small amounts of boron compounds play a role strengthening the cell walls of all plants, thus making boron necessary in soils. Experiments indicate a role for boron as an ultratrace element in animals. Deficiency of boron in rats has been shown to result in poor coat or hair quality.
Borates have low toxicity for mammals (similar to table salt), but are more toxic to arthropods and have been used as insecticides. Boric acid is mildly antimicrobial, and a natural, boron-containing, organic antibiotic does exist.
Some signaling compounds of boron which are used for bacterial cell to cell communication (known as "quorum sensing") have been discovered recently. This signaling system is used as a response to the local population density of bacterial cells, and mediates processes such as biofilm formation, virulence, and antibiotic resistance. A known example of a boron-containing quorum sensing signaling molecule is Autoinducer-2 (AI-2).
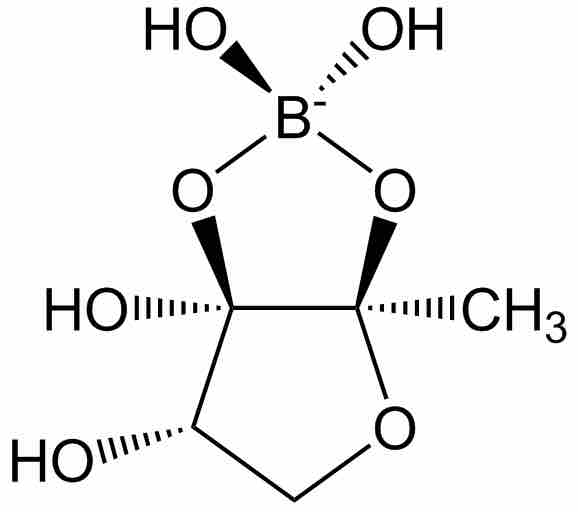
Autoinducer-2
Autoinducer-2 (AI-2) is one of the few known biomolecules containing the element Boron. AI-2 is used by many types of bacteria as a signaling molecule mediating quorum sensing.