Borates are the name for a large number of boron-containing oxoanions. The term may also more loosely refer to chemical compounds which contain borate anions. The simplest borate anion, BO33-, has a trigonal planar structure and is analogous to the carbonate anion CO32-, with which it is isoelectronic. Larger borates are composed of trigonal planar BO3 or tetrahedral BO4 structural units, joined together via shared oxygen atoms; these may be cyclic or linear in structure.
Borates in Nature
Borates are the form in which boron most often occurs in nature, mainly as borate minerals and borosilicates. The incomplete octet means that Borates act as Lewis acids. When a trigonal boron atom accepts a pair of electrons from a Lewis base, it adopts a tetrahedral configuration (sp3), and the octet rule is satisfied. Both trigonal and tetrahedral units can co-exist in a complex borate, such as the anion in borax.
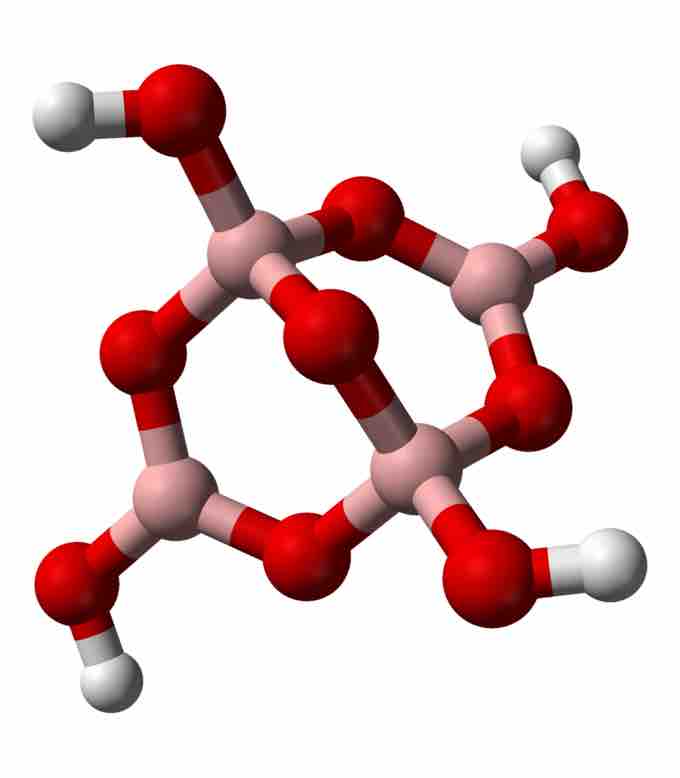
Tetraborate
Tetraborate (borax) ion structure. Pink: boron; red: oxygen; white: hydrogen.
Boron Monoxide
Boron monoxide (B2O) is another chemical compound of boron and oxygen. Two experimental studies have proposed the existence of diamond-like and graphite-like B2O, as for boron nitride and carbon solids. A later, systematic, experimental study of the boron oxide phase diagram suggests that B2O is unstable. The instability of the graphite-like B2O phase was also predicted theoretically.
Boron Trioxide
Boron trioxide (or diboron trioxide) is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. Boron trioxide is produced by treating borax with sulfuric acid in a fusion furnace.
Boron Suboxide
Boron suboxide (chemical formula B6O) is a solid compound containing six boron atoms and one oxygen atom. Due to its short interatomic bond lengths and strongly covalent character, B6O displays a range of outstanding physical and chemical properties such as great hardness (close to that of rhenium diboride and boron nitride), low mass density, high thermal conductivity, high chemical inertness, and excellent wear resistance. B6O can be synthesized by reducing B2O3 with boron or by oxidation of boron with zinc oxide or other oxidants.