Recall that in the periodic table, each row is called a period. The rows are aligned in such a way that the elements in each vertical column share certain characteristics. Each of the columns of the periodic table is called a group. Chemists have long found it convenient to refer to the elements of different groups, and in some cases of spans of groups, by the names shown in the table. Keep in mind that group names can give clues about the elements' metallic properties.
Trends in the periodic table
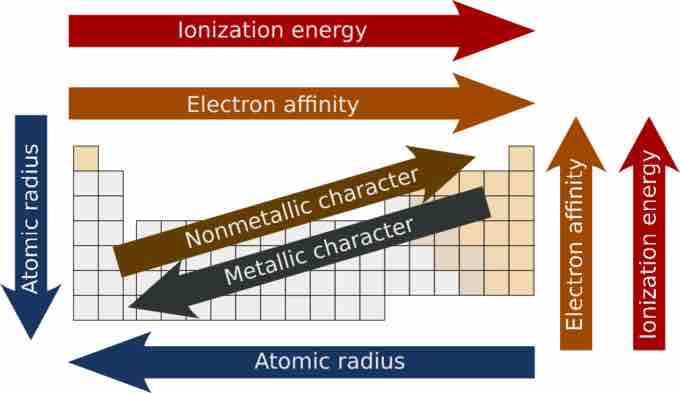
Families of the periodic table are often grouped by metallic properties.
When two elements are joined in a chemical bond, the element that attracts the shared electrons more strongly has more electronegativity. Elements with low electronegativity tend to have more metallic properties. So, the metallic properties of elements tends to decrease across a period and increase down a group. The fact that the metallic elements are found on the left side of the periodic table offers an important clue to the nature of how they bond together to form solids. These elements all possess low electronegativities and readily form positive ions.
Metals tend to form positive ions, and like charges repel, so how do metal atoms stay bonded together in a solid? The simplest conception of metals is a lattice of positive ions immersed in a "sea of electrons" that can migrate freely throughout the solid. In effect, the electropositive nature of the metallic atoms allows their valence electrons to exist as a mobile fluid. This results in their high electrical conductivities. Because each ion is surrounded by the electron fluid in all directions, the bonding has no directional properties; this accounts for the high malleability and ductility of metals.