A chemical equation is a visual representation of a chemical reaction. A typical chemical equation follows the form
where an arrow separates the reactants on the left and the products on the right. The coefficients before the reactants and products are their stoichiometric values.
Calculating the Mass of Reactants & Products
One may need to compute the mass of a reactant or product under certain reaction conditions. To do this, it is necessary to ensure that the reaction is balanced. The ratio of the coefficients of two of the compounds in a reaction (reactant or product) can be viewed as a conversion factor and can be used to facilitate mole-to-mole conversions within the reaction. It is not possible to directly convert from the mass of one element to the mass of another. Therefore, for a mass-to-mass conversion, it is necessary to first convert one amount to moles, then use the conversion factor to find moles of the other substance, and then convert the molar value of interest back to mass.
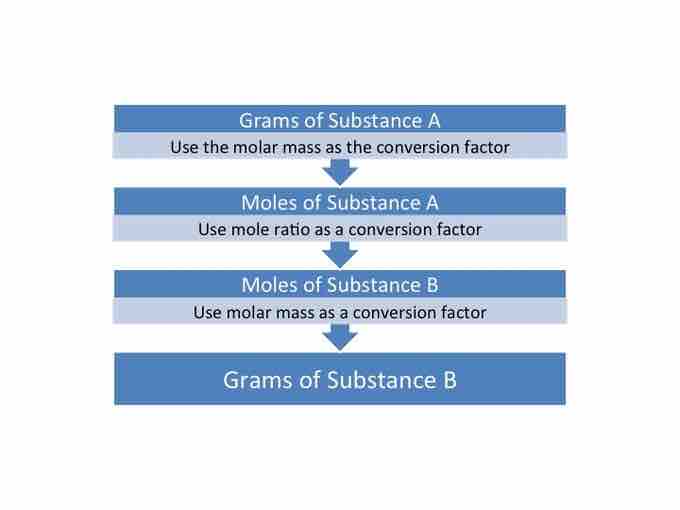
Mass to mass conversions
A chart detailing the steps that need to be taken to convert from the mass of substance A to the mass of substance B.
Example
This can be illustrated by the following example, which calculates the mass of oxygen needed to burn 54.0 grams of butane (C4H10). The balanced equation is:
Because there is no direct way to compare the mass of butane to the mass of oxygen, the mass of butane must be converted to moles of butane:
With the number of moles of butane equal to 54 grams, it is possible to find the moles of O2 that can react with it. Taking coefficients from the reaction equation (13 O2 and 2 C4H10), the molar ratio of O2 to C4H10 is 13:2.
This last equation shows that 6.05 moles of O2 can react with 0.929 moles of C4H10. The molar amount of O2 can now be easily converted back to grams of oxygen:
In summary, it was impossible to directly determine the mass of oxygen that could react with 54.0 grams of butane. But by converting the butane mass to moles (0.929 moles) and using the molar ratio (13 moles oxygen : 2 moles butane), one can find the molar amount of oxygen (6.05 moles) that reacts with 54.0 grams of butane. Using the molar amount of oxygen, it is then possible to find the mass of the oxygen (193 g).