Concept
Version 8
Created by Boundless
Major Features of a Phase Diagram
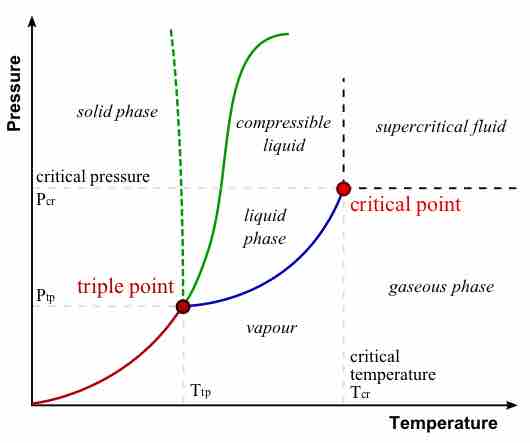
Phase Diagram
In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries. The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa). The dotted green line is meant to replace the solid green line in the corresponding phase diagram of water. It illustrates water's anomalous behavior.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: