Laminar Flow
When fluids flow smoothly through a tube or pipe, the motion can be thought of as consisting of layers, or lamina. This is called laminar flow (also known as streamlined flow), and the velocity of the fluid's flow varies from close to zero near the pipe's boundaries to its greatest in the center.
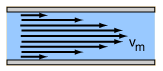
Laminar flow
Velocity of a fluid's layers, or lamina, during smooth flow. The velocity is greatest at the center of the tube.
If we consider one of those layers as a moving plate within the fluid, then the velocity is highest right underneath the moving plate but gets progressively smaller as we move away from the plate in a direction perpendicular to the flow.
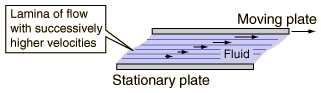
Moving plate in a fluid
The velocity of the liquid (in the x-direction) is highest near the moving plate and gets progressively smaller as we move away from the plate in the perpendicular, or y-, direction. Note the magnitude of the velocity vectors for layers increasingly away from the moving plate.
Description of Viscosity
The force required to move the plate at a constant speed is directly proportional to the area of the plate and to the fluid's velocity gradient as we move at a greater perpendicular distance from the plate (meaning how fast the velocity of the layers is changing as we move away from the plate). This implies the following quantitative relationship:
where F is the force required to move the plate (at constant speed), A is the area of the plate,
Viscosity is highly dependent on temperature, and therefore, when the value of the quantity is reported, the temperature at which the measurement was made must be included.
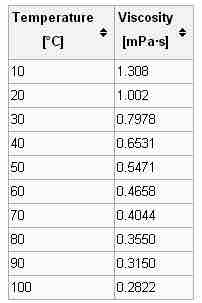
Viscosity dependence on temperature
The viscosity of water varies greatly over the temperature range of the liquid form.
Experimental Measurements
Viscosity can be measured by observing the time required for a given volume of liquid to flow through the narrow part of a viscometer tube, a special instrument used for such measurements.
There are various types of viscometers and rheometers. A rheometer is used for fluids that cannot be defined by a single value of viscosity. They require more parameters to be set and measured than is the case for a viscometer. Close temperature control of the fluid is essential to acquire accurate measurements, particularly in materials like lubricants, whose viscosity can double with a change of only 5 °C.
When measuring viscosity, strain is applied at a certain rate, called shear rate. The resulting stress is measured as deformation, in the case of liquid, as the ease of flow. There are several ways in which the stress changes with the strain. In simple linear systems, it is directly proportional and sometimes constant. In other systems, it increases or decreases with shear rate.
Fluids that display a constant viscosity over a range of shear rates are called Newtonian, while those with a non-constant viscosity are non-Newtonian. In the latter category, fluids that decrease in viscosity as shearing rate increases are called shear thinning (examples include toothpaste and house paint), and those that increase in viscosity as shearing rate increases are called shear thickening.

Viscosity plot
This figure shows the relative relationships between shear stress and shear strain for a variety of materials.
Intermolecular Forces Affect the Viscosity of a Substance
The viscosity of a substance is related to the strength of the intermolecular forces acting between its molecular units. In the case of water, these forces are primarily due to hydrogen bonding. Liquids such as syrups and honey are much more viscous because the sugars they contain are studded with hydroxyl groups (–OH). They can form multiple hydrogen bonds with water and with each other, producing a sticky disordered network which makes flow much more difficult (resulting in high viscosity).
In much of our daily experience, we act as viscometers, sensing the viscosity of liquids and assessing their fitness for a particular purpose. For example, some people add cream and sugar to coffee or tea not only for the taste but for what they term the mouth feel. In this case, we are forcing the fluid through a small gap between our tongue and palate, sensing the thickness and smoothness. Cream and sugar add particulate solids to the watery tea and coffee, which add to the viscosity of the solution.
In another setting, we judge the quality of a sauce by the way it flows and adheres to certain foods - salad dressing to lettuce, jam or jelly to toast, ketchup or mayonnaise to fried foods - the flow and cohesion are related to the viscosity. If you were to place honey or corn syrup in the refrigerator, it thickens considerably relative to its thickness at room temperature. We can see that viscosity is highly dependent on temperature.
In everyday terms, viscosity is described as thickness or internal friction within the substance. Therefore, we say water is thin, having a low viscosity, while honey is thick, having a high viscosity. When a fluid is less viscous, it flows more easily.