SI Units
The International System of Units (abbreviated SI from the French Système International d'Unités) is the basis of the metric system. The SI was established in 1960 and is based on the metre-kilogram-second system rather than the centimetre-gram-second system. The units are divided into two classes: base units and derived units. There are seven base units, each representing a different kind of physical quantity.
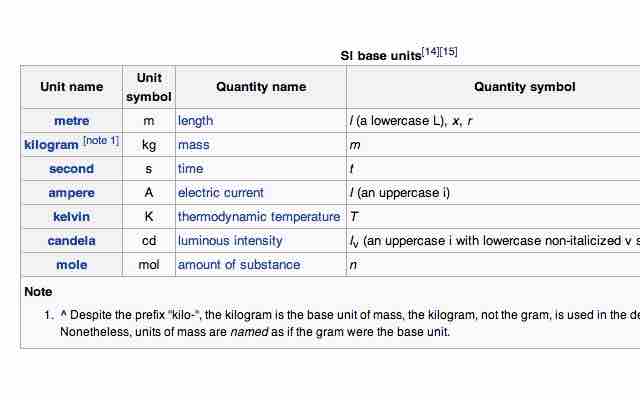
SI base units
The seven SI base units.
Derived Units
Derived units are unlimited in number and are formed by multiplying and dividing the seven base units and other derived units; for example, the SI derived unit of speed is meters per second, m/s. Some derived units have special names; for example, the unit of resistance, the ohm (Ω), is uniquely defined by the following relation:
This follows the definition of electrical resistance.
Pressure, the effect of a force applied to a surface, is a derived unit. The unit of pressure in the SI system is the pascal (Pa), defined as the force of one newton per square meter:
In chemistry, it is more common to express pressures in units of atmospheres or torr:
1 atm = 101325 Pa = 760 torr
Torr and millimeters of mercury (mm Hg, defined as a one millimeter difference in the height of a mercury barometer at 0°C) are nearly equivalent. Another unit of pressure used in meteorology is the bar:
1 bar = 105 N/m2 = 750.06 torr = 0.987 atm.
Since the quantities measured can have such a wide range, a standardized prefix system has been set in place.
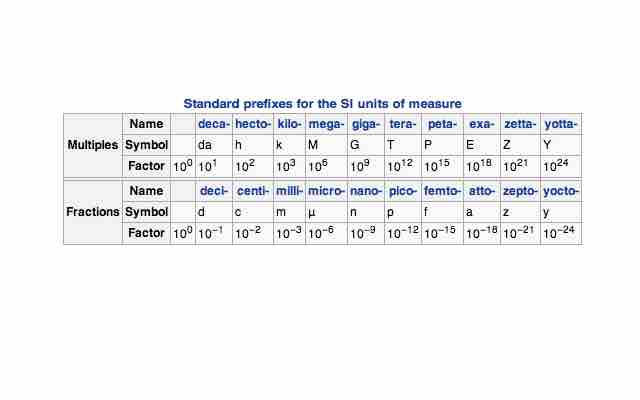
Standard prefixes for SI units
A prefix may be added to a unit's name to describe a multiple of the original unit.
This allows us to easily write out very small and very large numbers, such as 1 mPa (millipascal, 10-3) or 1 GPa (gigapascal, 109, e.).
Pressure can be represented by many different units and prefixes. When performing pressure calculations, it is important to ensure that all dimensions are in the same unit system.
Example 1
On a given day, the atmospheric pressure is 770 mm Hg. What is the pressure in pascals?
The pressure is 102.6 pascals.