Lead Batteries
A lead storage battery, also known as a lead-acid battery, is the oldest type of rechargeable battery and one of the most common energy storage devices. These batteries were invented in 1859 by French physicist Gaston Planté, and they are still used in a variety of applications. Most people are accustomed to using them in vehicles, where they have the ability to provide high currents for cranking power.
Although the batteries are reliable, they have a limited life, are heavy to ship, and contain toxic materials that require specific removal methods at the end of their useful life. Lead-acid batteries have moderate power density and good response time. Depending on the power conversion technology incorporated, batteries can go from accepting energy to supplying energy instantaneously. Lead-acid batteries are affected by temperature and must be maintained in order to achieve maximum life expectancy.
Designing a Lead Battery
In Planté's design of the lead-acid cell, the positive and negative plates were made out of two spirals of lead foil, separated with a sheet of cloth, and coiled up. The cells initially had low capacity. A slow process of "forming" was required to corrode the lead foils, creating lead dioxide on the plates and roughening them to increase surface area. Planté plates are still used in some stationary applications, where the plates are mechanically grooved to increase surface area.
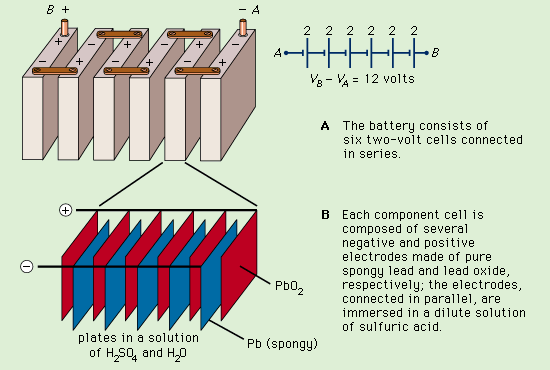
Lead storage battery
A diagram showing how a lead storage battery consists of six two-volt cells connected in series. The make up of each cell is also shown.
Camille Alphonse Faure's pasted-plate construction is typical of automotive batteries today. Each plate consists of a rectangular lead grid. The holes of the grid are filled with a paste of red lead and 33 percent dilute sulfuric acid. This porous paste allows the acid to react with the lead inside the plate, which increases the surface area. Once dry, the plates are stacked with suitable separators and inserted into the battery container. An odd number of plates is usually used, with one more negative plate than positive. Each alternate plate is connected.
The paste contains carbon black, barium sulfate, and lignosulfonate. The barium sulfate acts as a seed crystal for the lead-to-lead sulfate reaction. The lignosulfonate prevents the negative plate from forming a solid mass during the discharge cycle, and instead enables the formation of long needle-like crystals. Carbon black counteracts the effect of inhibiting formation caused by the lignosulfonates.
Discharge Chemistry
In the discharged state, both the positive and negative plates become lead(II) sulfate (PbSO4). The electrolyte loses much of its dissolved sulfuric acid and becomes primarily water. The discharge process is driven by the conduction of electrons from the negative plate back into the cell at the positive plate in the external circuit.
Negative plate reaction: Pb(s) + HSO4-(aq) → PbSO4(s) + H+(aq) + 2e-
Positive plate reaction: PbO2(s) + HSO4-(aq) + 3H+(aq) + 2e- → PbSO4(s) + 2H2O(l)
Combining these two reactions, one can determine the overall reaction:
Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4-(aq) → 2PbSO4(s) + 2H2O(l)
Charge Chemistry
This type of battery can be recharged. In the charged state, each cell contains negative plates of elemental lead (Pb) and positive plates of lead(IV) oxide (PbO2) in an electrolyte of approximately 4.2 M sulfuric acid (H2SO4). The charging process is driven by the forcible removal of electrons from the positive plate and the forcible introduction of them to the negative plate by the charging source.
Negative plate reaction: PbSO4(s) + H+(aq) + 2e- → Pb(s) + HSO4-(aq)
Positive plate reaction: PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4-(aq) + 3H+(aq) + 2e-
Combining these two reactions, the overall reaction is the reverse of the discharge reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4-(aq)
Notice how the charging reaction is the exact opposite of the discharge reaction.