Catalysts are compounds that, when added to chemical reactions, reduce the activation energy and increase the reaction rate. The amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Catalysts lower the energy required to reach the transition state of the reaction, allowing more molecular interactions to achieve that state. However, catalysts do not affect the degree to which a reaction progresses. In other words, though catalysts affect reaction kinetics, the equilibrium state remains unaffected.
Catalysts can be classified into two types: homogeneous and heterogeneous. Homogeneous catalysts are those which exist in the same phase (gas or liquid) as the reactants, while heterogeneous catalysts are not in the same phase as the reactants. Typically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixture.
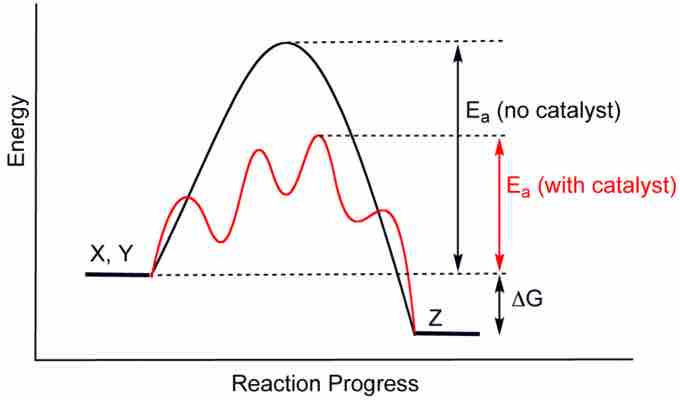
Catalysis
Note the lowered activation energy of the catalyzed pathway.
Examples of Homogeneous Catalysts
Acid catalysis, organometallic catalysis, and enzymatic catalysis are examples of homogeneous catalysis. Most often, homogeneous catalysis involves the introduction of an aqueous phase catalyst into an aqueous solution of reactants. In such cases, acids and bases are often very effective catalysts, as they can speed up reactions by affecting bond polarization.
An advantage of homogeneous catalysis is that the catalyst mixes into the reaction mixture, allowing a very high degree of interaction between catalyst and reactant molecules. However, unlike with heterogeneous catalysis, the homogeneous catalyst is often irrecoverable after the reaction has run to completion.
Homogeneous catalysts are used in variety of industrial applications, as they allow for an increase in reaction rate without an increase in temperature.