Concept
Version 14
Created by Boundless
Double and Triple Covalent Bonds
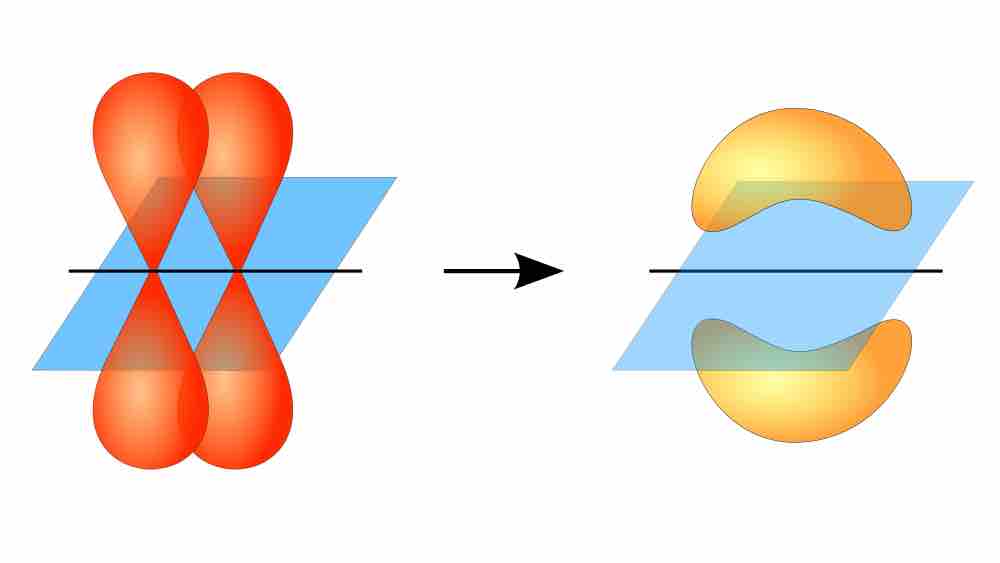
Pi bond formation
Overlap between adjacent unhybridized p orbitals produces a pi bond. The electron density corresponding to the shared electrons is not concentrated along the internuclear axis (i.e., between the two atoms), unlike in sigma bonds.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: