Concept
Version 18
Created by Boundless
Introduction to Lewis Structures for Covalent Molecules
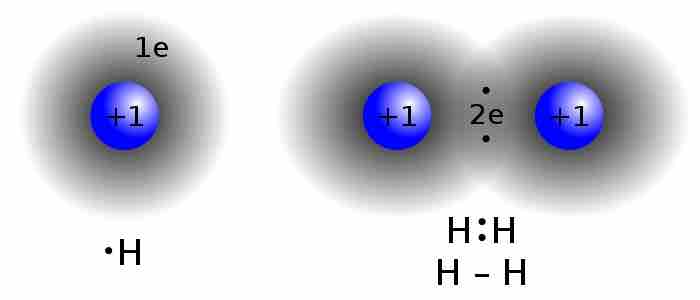
Lewis structure of diatomic hydrogen
This is the process through which the H2 molecule is formed. Two H atoms, each contributing an electron, share a pair of electrons. This is known as a 'single covalent bond.' Notice how the two electrons can be found in a region of space between the two atomic nuclei.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: