Concept
Version 18
Created by Boundless
Introduction to Lewis Structures for Covalent Molecules
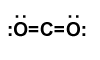
Final Lewis structure for carbon dioxide
Covalent bonds are indicated as dashes and lone pairs of electrons are shown as pairs of dots. in carbon dioxide, each oxygen atom has two lone pairs of electrons remaining; the covalent bonds between the oxygen and carbon atoms each use two electrons from the oxygen atom and two from the carbon.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Lewis carbon dioxide."
http://commons.wikimedia.org/wiki/File:Lewis_carbon_dioxide.gif
Wikimedia Commons
Public domain.