Section 3
The Structure of the Atom
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
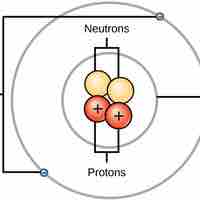
Overview of Atomic Structure
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
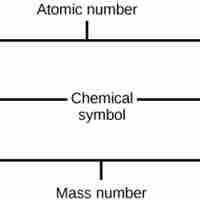
Atomic Number and Mass Number
The atomic number is the number of protons in an element, while the mass number is the number of protons plus the number of neutrons.

Isotopes
Isotopes are various forms of an element that have the same number of protons, but a different number of neutrons.