An atom is a basic unit of matter that consists of a dense nucleus composed of positively charged protons and neutral neutrons, which is surrounded by a cloud of negatively charged electrons. If an atom has the same number of protons and electrons, it is electronically neutral. However, if the total number of electrons does not equal the number of protons, the atom has a net electrical charge.
Any atom or molecule with a net charge, either positive or negative, is known as an ion. An ion consisting of a single atom is a monoatomic ion; an ion consisting of two or more atoms is referred to as a polyatomic ion. The positive electric charge of a proton is equal in magnitude to the negative charge of an electron; therefore, the net electric charge of an ion is equal to its number of protons minus its number of electrons.
Ions are highly reactive species. They are generally found in a gaseous state and do not occur in abundance on Earth. Ions in the liquid or solid state are produced when salts interact with their solvents. They are repelled by like electric charges and are attracted to opposite charges.
Types of Ions
There are specialized types of ions. Anions have more electrons than protons and so have a net negative charge. Cations have more protons than electrons and so have a net positive charge. Zwitterions are neutral and have both positive and negative charges at different locations throughout the molecule. Anions are generally larger than the parent molecule or atom, because the excess electrons repel each other and add to the physical size of the electron cloud. Cations are generally smaller than their parent atom or molecule due to the smaller size of their electron clouds.
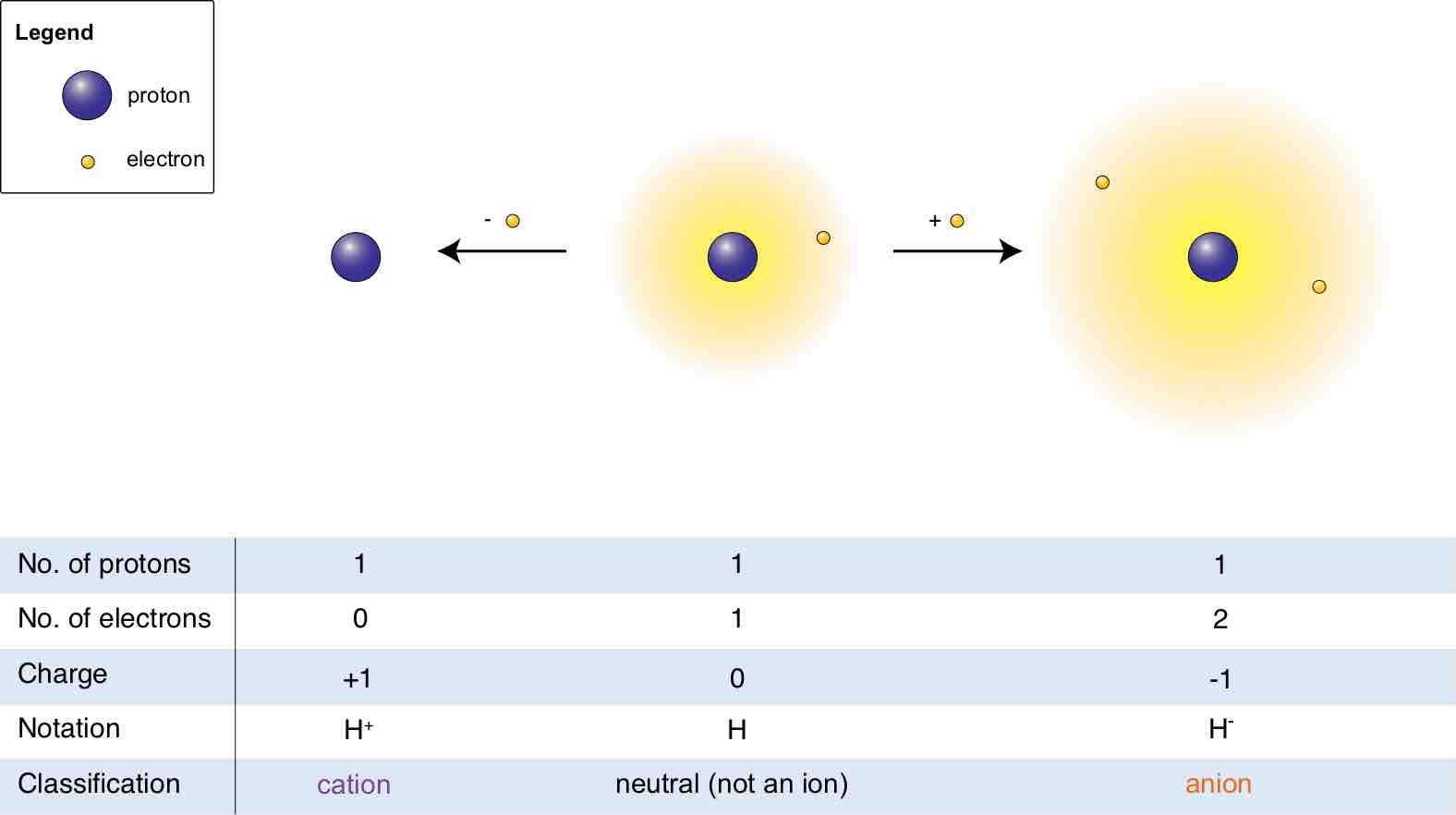
Hydrogen ions
The relationship between a molecule, its cation, and its anion is shown.
An ion is denoted by writing its net negative charge in superscript immediately after the chemical structure for the atom/molecule. Conventionally the net charge is written with the magnitude before the sign; the magnitude of singly charged molecules/atoms is generally omitted. Monoatomic ions are sometimes also represented by Roman numerals, which designate the formal oxidation state of the element, whereas the superscripted numerals denote the net charge. For example, Fe2+ can be referred to as Fe(II). These representations can be thought of as equivalent for monoatomic ions, but the Roman numerals cannot be applied to polyatomic ions.
Forming Ions
Ions can be formed by ionization, which is the process of a neutral atom losing or gaining electrons. Generally, the electrons are either added to or lost from the valence shell of an atom; the inner-shell electrons are more tightly bound to the positively charged nucleus and so do not participate in this type of chemical interaction.
Ionization generally involves a transfer of electrons between atoms or molecules. The process is motivated by the achievement of more stable electronic configurations, such as the octet rule, which states that most stable atoms and ions have eight electrons in their outermost (valence) shell. Polyatomic and molecular ions can also be formed, generally by gaining or losing elemental ions, such as H+, in neutral molecules. Polyatomic ions are generally very unstable and reactive.
An common example of an ion is Na+. Sodium has a +1 charge because sodium has eleven electrons. However, according to the octet rule, sodium would be more stable with 10 electrons (2 in its inner most shell, 8 in its outermost shell). Therefore, sodium tends to lose an electron to become more stable. On the other hand, chlorine tends to gain an electron to become Cl-. Chlorine naturally has 17 electrons but it would be more stable with 18 electrons (2 in its inner most shell, 8 in its second shell, and 8 in its valence shell). Therefore, chlorine will take an electron from another atom to become negatively charged.