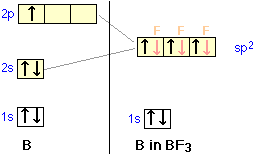
Boron trifluoride (BF3) has a boron atom with three outer-shell electrons in its normal or ground state, as well as three fluorine atoms, each with seven outer electrons. One of the three boron electrons is unpaired in the ground state. In order to explain the bonding, the 2s orbital and two of the 2p orbitals (called sp2 hybrids) hybridize; one empty p-orbital remains.
Boron configuration diagram
One of the three boron electrons is unpaired in its ground state. The atomic s- and p-orbitals in boron's outer shell mix to form three equivalent hybrid orbitals. These particular orbitals are called sp2 hybrids, meaning that this set of orbitals derives from one s- orbital and two p-orbitals of the free atom.
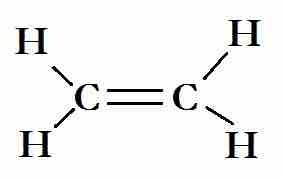
The Lewis structure for ethene
The carbon atoms are sp2 hybridized. Two sp2 hybrids bond with the hydrogen atoms, and the other forms a sigma bond with the other carbon atom. The p-orbitals that are unused by the carbon atoms in the hybridization overlap to form the C=C.
sp2 Hybridization in Ethene and the Formation of a Double Bond
Ethene (C2H4) has a double bond between the carbons. In this case, carbon will sp2 hybridize; in sp2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp hybrid orbitals with one p-orbital remaining. The three hybridized orbitals explain the three sigma bonds that each carbon forms.
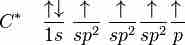
sp2 hybridization in ethene
In sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one p-orbital remaining.
The two carbon atoms form a sigma bond in the molecule by overlapping two sp2 orbitals. Each carbon atom forms two covalent bonds with hydrogen by s–sp2 overlap, all with 120° angles. The pi bond between the carbon atoms perpendicular to the molecular plane is formed by 2p–2p overlap.
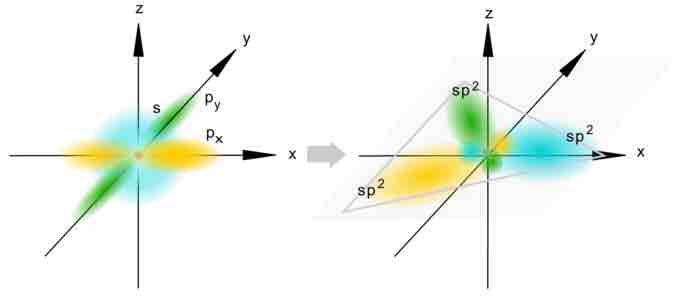
Formation of sp2 hybrid orbitals
This illustration shows how an s-orbital mixes with two p orbitals to form a set of three sp2 hybrid orbitals. Notice again how the three atomic orbitals yield the same number of hybrid orbitals.